TLX3在小脑发育过程中调控CGN祖细胞增殖,其功能障碍可导致自闭症
IF 4.6
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
Tlx3是兴奋性神经元命运规范的主调节因子,主要在有丝分裂后细胞中发挥作用。虽然我们之前已发现 Tlx3 在小脑增殖的颗粒神经元祖细胞(GNPs)中表达,但其主要作用尚不清楚。在这里,我们证明了 GNPs 中 Tlx3 的功能障碍会通过调节抗增殖基因而显著减少其增殖。因此,GNPs 生成的改变导致了小脑发育不良、形态缺陷、颗粒神经元-浦肯野比例失调以及小脑突触连接异常。这种小脑平衡的改变表现为小鼠典型的自闭症样行为,并伴有运动和社会功能障碍。我们还发现,在人类自闭症谱系障碍(ASD)病例中存在未定性突变的 TLX3 变体。总之,我们的研究确定了 Tlx3 是参与发育 GNP 的关键基因,从早期发育阶段就缺失 Tlx3 会导致自闭症。本文章由计算机程序翻译,如有差异,请以英文原文为准。
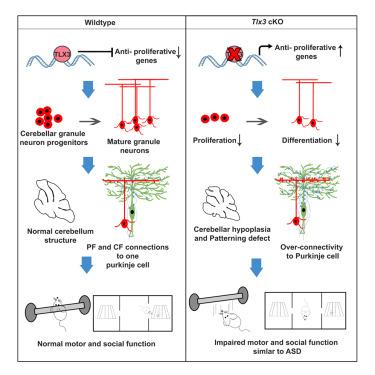
TLX3 regulates CGN progenitor proliferation during cerebellum development and its dysfunction can lead to autism
Tlx3, a master regulator of the fate specification of excitatory neurons, is primarily known to function in post-mitotic cells. Although we have previously identified TLX3 expression in the proliferating granule neuron progenitors (GNPs) of cerebellum, its primary role is unknown. Here, we demonstrate that the dysfunction of Tlx3 from the GNPs significantly reduced its proliferation through regulating anti-proliferative genes. Consequently, the altered generation of GNPs resulted in cerebellar hypoplasia, patterning defects, granule neuron-Purkinje ratio imbalance, and aberrant synaptic connections in the cerebellum. This altered cerebellar homeostasis manifested into a typical autism-like behavior in mice with motor, and social function disabilities. We also show the presence of TLX3 variants with uncharacterized mutations in human cases of autism spectrum disorder (ASD). Altogether, our study establishes Tlx3 as a critical gene involved in developing GNPs and that its deletion from the early developmental stage culminates in autism.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


