柠檬酸盐络合法制备 CuO-ZnO-ZrO2-Al2O3 催化剂用于 CO2 加氢制甲醇的溶剂效应
IF 6.7
1区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
摘要
二氧化碳加氢制甲醇是实现碳中和的有效方法之一,而铜基催化剂是该反应的极佳催化剂。然而,在开发高活性、高稳定性的铜基催化剂时,溶剂效应往往被忽视。本研究考察了柠檬酸盐络合法制备的 CuO-ZnO-ZrO2-Al2O3 (CZZA) 催化剂的溶剂效应。研究发现,乙醇作为溶剂可显著提高 CZZA 催化剂的催化性能。该研究强调了乙醇在改善活性成分 Cu0 的分散和促进氧空位的形成方面的作用,从而提高了整体催化效率。原位 DRIFTS 的结果表明,CZZA 催化剂通过甲酸盐途径实现了二氧化碳加氢,并证明氢气溢出至关重要。本文提出的简单改进方法可有效改善金属氧化物的分散性,增强活性物种 Cu 与金属氧化物之间的相互作用,为设计 CO2 加氢制甲醇的高效催化剂提供了依据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
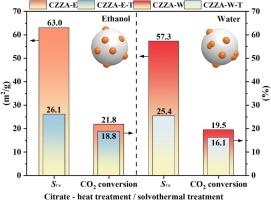
Solvent effects on the preparation of CuO-ZnO-ZrO2-Al2O3 catalyst by citrate complexing method for CO2 hydrogenation to methanol
CO2 hydrogenation to methanol is one of the effective ways to achieve carbon neutrality, and copper-based catalysts are excellent catalysts for this reaction. However, the solvent effect is often overlooked when developing highly active and stable copper-based catalysts. In this study, the solvent effect of CuO-ZnO-ZrO2-Al2O3 (CZZA) catalysts prepared by a citrate complexing method was investigated. It was found that ethanol as a solvent could significantly improve the catalytic performance of CZZA catalyst. The study highlights the role of ethanol in improving the dispersion of the active component Cu0 and promoting the formation of oxygen vacancies, thereby improving the overall catalytic efficiency. The results of in-situ DRIFTS showed that the CZZA catalyst achieved CO2 hydrogenation through the formate pathway and demonstrated that hydrogen overflow is crucial. The simple improvement method proposed in this work can effectively improve the dispersion of metal oxides and enhance the interaction between the active species Cu and metal oxides, providing a basis for the design of efficient catalysts for CO2 hydrogenation to methanol.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fuel
工程技术-工程:化工
CiteScore
12.80
自引率
20.30%
发文量
3506
审稿时长
64 days
期刊介绍:
The exploration of energy sources remains a critical matter of study. For the past nine decades, fuel has consistently held the forefront in primary research efforts within the field of energy science. This area of investigation encompasses a wide range of subjects, with a particular emphasis on emerging concerns like environmental factors and pollution.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


