将二氧化碳高选择性电化学转化为 CO 的纳米结构 Zn 基电催化剂的战略方法
IF 7.5
1区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
摘要
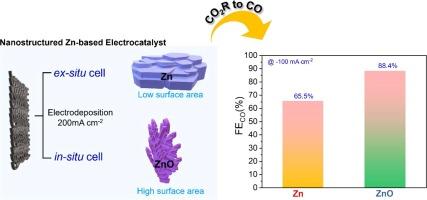
二氧化碳(CO2)的电还原直接促进了间歇性回收利用向高附加值化学品和燃料的转化,是解决二氧化碳排放造成的能源和环境危机的有利策略。因此,有必要开发高活性、高选择性的新兴电催化剂,以进行二氧化碳到一氧化碳(CO)的电化学还原(ECR)。本研究发现了一种低成本、富集于地球且无毒的锌(Zn)基电催化剂,它是一种很有前景的 CO 选择性电催化剂。该研究采用简便的电沉积(ED)方法,在两种不同的配置中合成了锌基电催化剂:原位电池和原位电池。原位电池采用传统的三电极系统,产生的电催化剂称为 Zn87。相反,原位电池采用气体扩散电极 (GDE),形成的电催化剂被认定为 ZnO52。根据 XRD 分析对电催化剂进行的相定量,Zn 电催化剂显示出 Zn(87%)和 ZnO(13%)。而氧化锌电催化剂则显示出 Zn(48%)和 ZnO(52%)。因此,根据主要相位值,电催化剂的名称被调整为:Zn 为 Zn87%,ZnO 为 ZnO52%。在这里,由原位电池生长的 ZnO52 电催化剂显示出较大的电活性表面积,在 -0.87 V 电压下与可逆氢电极(VRHE)相比,具有更高效的 CO 生产 ECR 活性,法拉第效率(FECO)为 88.4%。然而,通过原位电池生长的 Zn87 电催化剂在施加 100 mA cm-2 的电流密度时,在 -0.98 V 可逆氢电极 (VRHE) 下的 FECO 为 65.6%。因此,ZnO52 电催化剂电化学活性的增强可归因于其大表面积提供了巨大的吸附能力,而占主导地位的 Zn2+ 则创造了更多的活性位点。此外,电催化剂中的对应锌有助于稳定 ZnO 状态,避免在 CO2R 过程中将 ZnO 转化为金属锌。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A strategic approach for nanostructured Zn-based electrocatalyst for highly selective electrochemical conversion of CO2 to CO
Electroreduction of carbon dioxide (CO2) directly facilitates the conversion of intermittent recycling into value-added chemicals and fuel, presenting a favorable strategy to resolve the energy and environmental crises caused by CO2 emissions. Therefore, there is a necessity to develop highly active and selective emerging electrocatalysts to perform electrochemical CO2 reduction (ECR) to carbon monoxide (CO). This work identifies a low-cost, earth-abundant, and non-toxic Zinc (Zn)-based electrocatalyst as a promising electrocatalyst for CO selectivity. Zn-based electrocatalysts were synthesized using a facile electrodeposition (ED) method in two different configurations: an ex situ cell and an in situ cell. The ex situ cell utilized a conventional three-electrode system, yielding an electrocatalyst referred to as Zn87. Conversely, the in situ cell employed a gas diffusion electrode (GDE), resulting in the formation of an electrocatalyst identified as ZnO52. Based on the phase quantification of the electrocatalysts from XRD analysis, the Zn electrocatalyst shows Zn (87 %) and ZnO (13 %). Whereas, the ZnO electrocatalyst shows the Zn (48 %) and ZnO (52 %). Thus, based on the predominant phase values, the electrocatalysts name, Zn as Zn87%, and ZnO as ZnO52% respectively, were adapted. Here, the ZnO52 electrocatalysts, grown by the in situ cell, showed a large electroactive surface area, demonstrating more efficient ECR activity toward CO production with a Faradaic efficiency (FECO) of 88.4 % at –0.87 V versus reversible hydrogen electrode (VRHE). However, the Zn87 electrocatalyst, grown by the ex situ cell exhibited a FECO of 65.6 % at –0.98 VRHE at an applied current density of 100 mA cm−2. Accordingly, the enhanced electrochemical activity of the ZnO52 electrocatalyst can be attributed to the large surface area, which provides great adsorption capacity, and the predominant Zn2+ create more active sites. Additionally, the Zn counterpart of the electrocatalyst, helps to stabilize ZnO state from converting ZnO to metallic Zn during the CO2R performance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fuel
工程技术-工程:化工
CiteScore
12.80
自引率
20.30%
发文量
3506
审稿时长
64 days
期刊介绍:
The exploration of energy sources remains a critical matter of study. For the past nine decades, fuel has consistently held the forefront in primary research efforts within the field of energy science. This area of investigation encompasses a wide range of subjects, with a particular emphasis on emerging concerns like environmental factors and pollution.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


