二氧化硫诱导镍基材料失活的机理研究,用于二氧化碳捕集和甲烷化
IF 6.7
1区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
摘要
用于循环捕获二氧化碳和甲烷化的双功能材料(DFMs)在减缓全球气候变化和实现碳中和方面具有巨大潜力。然而,二氧化硫中毒导致的材料失活是其工业应用面临的一大挑战。在此,我们定制了一种镍基 DFM,并系统研究了硫中毒对二氧化碳吸附和原位转化的影响。实验结果表明,二氧化硫浓度、二氧化碳捕集能力和甲烷产量之间存在显著的反比关系。随着二氧化硫浓度从 100 ppmv 增加到 1000 ppmv,甲烷产量的下降率从 7.14% 急剧上升到 85.71%。物理化学特征表明,二氧化硫会在 DFM 表面积聚,最初形成亚硫酸盐,并在二氧化碳吸附过程中氧化成硫酸盐。此外,硫中毒会加速金属镍在循环反应后氧化成 Ni2+,从而抑制 DFM 的高温碱性位点和表面氧空位。原位 DRIFT 研究表明,沉积的硫酸盐在 340°C 的 H2 还原过程中保持稳定,有助于甲酸盐中间体的分解,最终导致甲烷产量下降。本文章由计算机程序翻译,如有差异,请以英文原文为准。
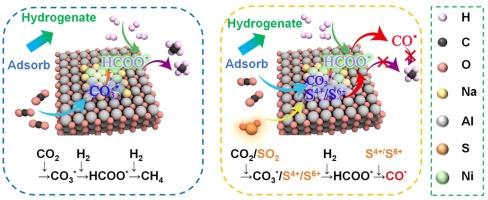
Mechanistic insights into SO2-induced deactivation of Ni-based materials for integrated CO2 capture and methanation
Dual functional materials (DFMs) for cyclic CO2 capture and methanation exhibit significant potential in mitigating global climate change and achieving carbon neutrality. However, material deactivation caused by SO2 poisoning presents a major challenge for its industrial applications. Herein, we tailored a kind of Ni-based DFM, and the sulfur poisoning effects on CO2 adsorption and in-situ conversion were systematically investigated. The experimental results reveal a striking inverse relationship between SO2 concentration, CO2 capture capacity, and methane yield. Increasing SO2 concentration promotes the form of stable sulfate species and undecomposable, lower CO2 capture capacity which further decreases methane yield with the rate of decrease in methane yield rising sharply from 7.14 % to 85.71 % as the SO2 concentration increases from 100 ppmv to 1000 ppmv, compared to the methane yield in the absence of SO2. Physicochemical characterizations demonstrate that SO2 accumulates on the surface of DFM, initially forming sulfite and oxidizing to sulfate during the CO2 adsorption process. Furthermore, sulfur poisoning accelerates the oxidation of metallic Ni to Ni2+ after cyclic reactions, which suppresses high-temperature basic sites and surface oxygen vacancies of DFM. In-situ DRIFT studies reveal that the deposited sulfate remains stable during H2 reduction at 340°C, contributing to the decomposition of formate intermediates and ultimately leading to a decrease in methane production.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fuel
工程技术-工程:化工
CiteScore
12.80
自引率
20.30%
发文量
3506
审稿时长
64 days
期刊介绍:
The exploration of energy sources remains a critical matter of study. For the past nine decades, fuel has consistently held the forefront in primary research efforts within the field of energy science. This area of investigation encompasses a wide range of subjects, with a particular emphasis on emerging concerns like environmental factors and pollution.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


