乙二醛在苯扎氯铵界面中与甲肟络合物的氧化作用:Raghavan 和 Srinivasan 动力学模型
IF 4.4
3区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
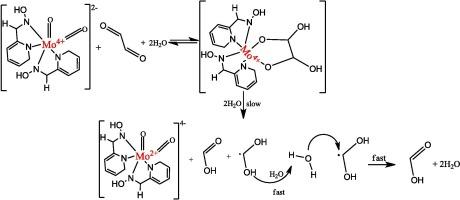
了解葡萄糖氧化产物(乙二醛)与金属有机络合物在生物环境中的相互作用对其使用至关重要。因此,我们采用假相方法,用分光光度法研究了乙二醛(Gyx)与 Mo-oxime 复合物(MC)的氧化作用。结果表明,水解、离子催化、中性原盐效应和自由基生成是 Gyx 氧化过程中的基本因素,而中间产物的形成则被排除在外。由于电荷中性反应物的参与,观察到 Gxy 的水解主动与 MC 发生反应,而没有电荷抑制,这就牵涉到中性原盐效应,即系统离子强度的变化保持氧化还原速率不变。当系统中加入 Mg2+ 离子添加剂时,电荷中性物种参与速率控制步骤,从而产生静电吸引,导致离子催化。Gxy 产生的自由基有助于甲酸的产生。Michaelis-Menten 型曲线截距为零,反应体系和 MC 的最大吸收波长无明显偏移,因此排除了中间物种的存在。热力学焓在氧化还原过程中起了重要作用,导致甲酸产物的产生。苯扎氯铵 (BZC) 的加入加速了 Gxy 的氧化,这也得到了 Raghavan 和 Srinivasan 模型的支持。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Oxidation of glyoxal with the Mo-oxime complex in a benzalkonium chloride interface: Raghavan and Srinivasan kinetic model
Gaining an understanding of the glucose oxidation product (glyoxal) interaction with the metal–organic complex in a biological environment is pivotal to its usage. Thus, the glyoxal (Gyx) oxidation with Mo-oxime complex (MC) is studied with the spectrophotometric method, following a pseudo-phase approach. The result highlights the inclusion of hydrolysis, ion catalysis, the neutral primary salt effect, and radical generation as the essential factors in Gyx oxidation, with the exclusion of intermediate species formation. The hydrolysis of Gxy is observed to actively engage MC without charge inhibition as charge-neutral reacting species are involved, thus, implicating neutral primary salt effect where the variation of ionic strength of the system keeps the redox rate unchanged. The involvement of charge-neutral specie at the rate controlling step encouraged electrostatic attraction when Mg2+ ion additive is incorporated into the system, leading to ion catalysis. The generation of free radical from Gxy aids the emergence of formic acid. The zero intercept of Michaelis–Menten type plot and the non-appreciable shift in the maximum absorption wavelength of the reaction system and MC rule-out the presence of intermediate species. The contribution of thermodynamic enthalpy is instrumental in the redox process, leading to the formic acid product. The inclusion of benzalkonium chloride (BZC) hastens the Gxy oxidation, which is supported by Raghavan and Srinivasan’s model.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry Communications
化学-无机化学与核化学
CiteScore
5.50
自引率
7.90%
发文量
1013
审稿时长
53 days
期刊介绍:
Launched in January 1998, Inorganic Chemistry Communications is an international journal dedicated to the rapid publication of short communications in the major areas of inorganic, organometallic and supramolecular chemistry. Topics include synthetic and reaction chemistry, kinetics and mechanisms of reactions, bioinorganic chemistry, photochemistry and the use of metal and organometallic compounds in stoichiometric and catalytic synthesis or organic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


