膨润土/二氧化钛量子点光降解和太阳能降解刚果红染料和工业染料废料的分解成本和回收工艺
IF 3.8
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
本研究介绍了一种新型膨润土-二氧化钛量子点(Bent/TQ)纳米复合材料的制备、表征和应用,用于高效光降解工业废水中常见的污染物刚果红染料。该复合材料是通过共沉淀法和煅烧法制备的,这有利于二氧化钛量子点在膨润土基质中的均匀分散。所制备的材料具有 205.45 m2/g 的高比表面积和 3.15 eV 的优化带隙,因此适用于可见光光催化。在氙灯和自然光照射下,对 Bent/TQ 的光催化效率进行了评估。复合材料的降解速率常数为 23.35 × 10-³ s-1,与纯 TiO2 量子点的降解速率常数(28.86 × 10-³ s-1)相当。机理研究表明,降解的主要活性物种是羟基自由基(-OH),它是通过光生电子-空穴对与 H2O 分子和分子氧的相互作用产生的。总有机碳 (TOC) 和化学需氧量 (COD) 分析用于评估复合材料的矿化效率。在自然阳光下照射 240 分钟后,Bent/TQ 催化剂的 COD 去除率达到 84.5%,TOC 降低率达到 76.2%。可重复使用性测试表明,光催化剂在连续 7 次循环后仍能保持约 80% 的原始效率,但由于颗粒在长时间光照下聚集,效率略有下降。经济分析表明,与传统的基于 TiO2 的系统相比,使用 Bent/TQ 纳米复合材料可降低 29.12% 的运营成本,使其成为大规模废水处理的一种具有成本效益的解决方案。这项工作凸显了 Bent/TQ 纳米复合材料作为可持续高效光催化剂用于环境修复的潜力,未来的工作目标是增强其长期稳定性,并将其适用范围扩大到更广泛的污染物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
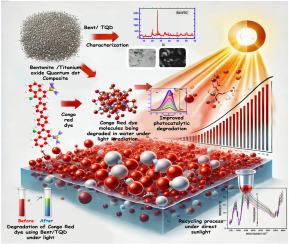
Breakdown cost and recycling processes of Bentonite/TiO2 quantum dots of photo and solar degradation of Congo Red dye and industrial dyes wastes
This research presents the preparation, characterization, and application of a novel bentonite- TiO2 quantum dot (Bent/TQ) nanocomposite for the efficient photodegradation of Congo Red dye, a common pollutant in industrial wastewater. The composite was prepared via a co-precipitation method followed by calcination, which facilitated the uniform dispersion of TiO2 quantum dots within the bentonite matrix. The resulting material exhibited a high specific surface area of 205.45 m2/g and an optimized band gap of 3.15 eV, making it suitable for visible light photocatalysis. The photocatalytic efficiency of Bent/TQ was evaluated under both xenon lamp irradiation and natural sunlight. The composite demonstrated a significant degradation rate constant of 23.35 × 10⁻³ s⁻1, which is comparable to that of pure TiO2 quantum dots (28.86 × 10⁻³ s⁻1). Mechanistic studies revealed that the primary active species responsible for the degradation were hydroxyl radicals (•OH), generated through the interaction of photogenerated electron-hole pairs with H2O molecules and molecular oxygen. Total Organic Carbon (TOC) and Chemical Oxygen Demand (COD) analyses were employed to assess the mineralization efficiency of the composite. The Bent/TQ catalyst achieved a COD removal efficiency of 84.5 % and a TOC reduction of 76.2 % after 240 min of irradiation under natural sunlight. Reusability tests indicated that the photocatalyst retained approximately 80 % of its original efficiency after seven consecutive cycles, though a slight decline was observed due to particle agglomeration under prolonged light exposure. The economic analysis suggested that the use of Bent/TQ nanocomposites could reduce operational costs by 29.12 % compared to conventional TiO2-based systems, making it a cost-effective solution for large-scale wastewater treatment. This work highlights the potential of Bent/TQ nanocomposites as sustainable and efficient photocatalysts for environmental remediation, with future efforts aimed at enhancing their long-term stability and expanding their applicability to a broader range of pollutants.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Optical Materials
工程技术-材料科学:综合
CiteScore
6.60
自引率
12.80%
发文量
1265
审稿时长
38 days
期刊介绍:
Optical Materials has an open access mirror journal Optical Materials: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
The purpose of Optical Materials is to provide a means of communication and technology transfer between researchers who are interested in materials for potential device applications. The journal publishes original papers and review articles on the design, synthesis, characterisation and applications of optical materials.
OPTICAL MATERIALS focuses on:
• Optical Properties of Material Systems;
• The Materials Aspects of Optical Phenomena;
• The Materials Aspects of Devices and Applications.
Authors can submit separate research elements describing their data to Data in Brief and methods to Methods X.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


