通过铜催化有机钛试剂与丙炔溴化物的 SN2′-取代反应高效合成二取代烯烃
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
报告了一种简单温和的有机钛试剂催化 SN2′型置换丙炔卤化物的方法。在二氯甲烷中,以 CuI (2 mol%)/MePPh2(4 mol%)为介导,在 40 °C 下催化 5 小时,丙炔基溴化物与有机钛试剂发生 SN2′型置换反应,得到了多取代烯。此外,带有萘基的二取代烯烃的选择性为 54-99%,分离产率为 43-83%。该工艺简单易行,为合成二取代烯烃衍生物提供了一种有效的方法。根据实验结果,提出了一种可能的催化循环。本文章由计算机程序翻译,如有差异,请以英文原文为准。
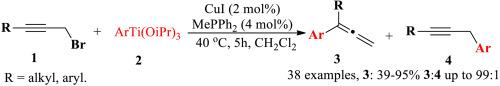
Highly efficient synthesis of di-substituted allenes via copper-catalyzed SN2′- substitution reaction of organotitanium reagents with propargyl bromides
A simple and mild catalytic SN2′-type displacement of propargyl halides with organotitanium reagents is reported. The SN2′-type displacement of propargyl bromides with organotitanium reagents mediated by CuI (2 mol%)/MePPh2(4 mol%) in dichloro methane afforded multi-substituted allenes in good yields (up to 95 %) at 40 °C for 5 h. The aryls bearing electron-donating or electron-withdrawing groups in aryltitanium reagents gave multi-substituted allenes in good yields. In addition, the di-substituted allenes bearing a naphthyl group was obtained in 54–99 % selectivities with isolated yields of 43–83 %. The process was simple and easily performed, and it provides an efficient method for the synthesis of di-substituted allene derivatives. On the basis of the experimental results, a possible catalytic cycle has been proposed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


