硫醇-烯类光刻胶在断层体积三维打印中的辐射抑制作用:从光流变学到可打印性
IF 5.1
3区 工程技术
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
断层体积三维打印技术的引入,使复杂的三维(生物)结构以低至 20 μm 的高精度被快速创建出来,从而彻底改变了制造和生物打印领域。尽管自由基抑制在体积三维打印中的作用已得到公认,但对抑制作用的研究却一直没有进展。本文提出了一个综合框架,以研究广泛报道的自由基抑制剂 TEMPO 在由异氰脲酸三烯丙酯(TTT)和季戊四醇四巯基丙酸酯(PETMP)组成的硫醇烯光交联光刻胶的体积三维打印性中的作用。通过光流变学测量、动力学建模、傅立叶变换红外光谱以及体积三维打印验证,阐明了抑制剂浓度、剪切模量的演变、光剂量与体积三维打印之间的关系。本研究的发现为预测断层体积三维打印光刻胶的最佳打印条件提供了可靠的方法,从而避免了大量的试验和错误。本文章由计算机程序翻译,如有差异,请以英文原文为准。
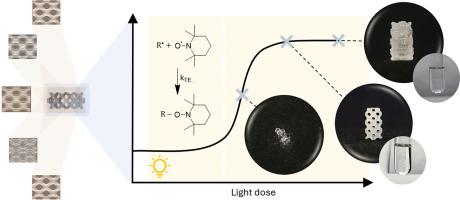
Radical inhibition in tomographic volumetric 3D printing for thiol-Ene photoresists: From Photorheology to printability
The introduction of tomographic volumetric 3D printing is revolutionizing the manufacturing and bioprinting landscape by enabling the rapid creation of complex three-dimensional (biological) structures with high precision down to 20 μm. Despite the recognized role of radical inhibition in volumetric 3D printing, a study of inhibition has remained elusive. Here, A comprehensive framework is presented to study the role of the widely reported radical inhibitor TEMPO in the volumetric 3D printability of a thiol-ene photocrosslinkable photoresist composed of triallyl isocyanurate (TTT) and pentaerythritol tetrakis(3-mercaptopropionate) (PETMP). Through photorheological measurements, kinetic modeling, FTIR spectroscopy, and validation via volumetric 3D printing, the relationship between inhibitor concentrations, the evolution of the shear moduli as well as the conversion as a function of light dose, and volumetric 3D printing is elucidated. The findings in this study provide a robust methodology for predicting optimal printing conditions of photoresists for tomographic volumetric 3D printing, obviating the need for extensive trial-and-error.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Reactive & Functional Polymers
工程技术-高分子科学
CiteScore
8.90
自引率
5.90%
发文量
259
审稿时长
27 days
期刊介绍:
Reactive & Functional Polymers provides a forum to disseminate original ideas, concepts and developments in the science and technology of polymers with functional groups, which impart specific chemical reactivity or physical, chemical, structural, biological, and pharmacological functionality. The scope covers organic polymers, acting for instance as reagents, catalysts, templates, ion-exchangers, selective sorbents, chelating or antimicrobial agents, drug carriers, sensors, membranes, and hydrogels. This also includes reactive cross-linkable prepolymers and high-performance thermosetting polymers, natural or degradable polymers, conducting polymers, and porous polymers.
Original research articles must contain thorough molecular and material characterization data on synthesis of the above polymers in combination with their applications. Applications include but are not limited to catalysis, water or effluent treatment, separations and recovery, electronics and information storage, energy conversion, encapsulation, or adhesion.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


