基于卟啉改性环氧胺网络的杀菌聚合物涂层
IF 4.5
3区 工程技术
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
研究首次证明,卟啉含量为 21.4 μM(0.002 wt%)的改性聚合物对金黄色葡萄球菌具有明显的杀菌活性。为了开发新型杀菌聚合物涂层,研究人员对双酚 A 二缩水甘油醚(DGEBA)和卟啉改性低聚二胺体系进行了研究。研究采用 Van Krevelen 的添加剂基团贡献法,通过实验和理论方法研究了卟啉在低聚物中的溶解度。研究获得了用不同游离基四芳基卟啉改性的完全固化环氧胺聚合物材料。通过热重法、差示扫描量热法、紫外-可见光谱法和荧光光谱法对这些材料进行了研究。所提出的形成改性环氧胺材料的方法可以保持卟啉的光物理特性,包括其光稳定性。在 4.28-21.4 μM 的浓度范围内添加改性剂可以保持聚合物基体的热物理和热化学特性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
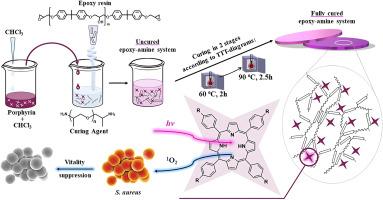
Biocidal polymer coatings based on porphyrin-modified epoxy-amine networks
For the first time, a pronounced biocidal activity of modified polymers with a porphyrin content of 21.4 μM (0.002 wt%) against Staphylococcus aureus was demonstrated. In order to develop new biocidal polymer coatings, a study was carried out on diglycidyl ether of bisphenol A (DGEBA) and porphyrin-modified oligomeric diamine systems. The study investigated the solubility of porphyrins in oligomers through experimental and theoretical means, using Van Krevelen's approach of additive group contributions. Fully cured epoxy-amine polymer materials modified with varied free-base tetraarylporphyrins were obtained. The materials were studied by means of thermogravimetry, differential scanning calorimetry, UV–Vis and fluorescence spectroscopy. The proposed approach to the formation of modified epoxy-amine materials allows preservation of the photophysical properties of porphyrins, including their photostability. The addition of modifiers in 4.28–21.4 μM range of concentrations makes it possible to keep the thermophysical and thermochemical properties of the polymer matrix.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Reactive & Functional Polymers
工程技术-高分子科学
CiteScore
8.90
自引率
5.90%
发文量
259
审稿时长
27 days
期刊介绍:
Reactive & Functional Polymers provides a forum to disseminate original ideas, concepts and developments in the science and technology of polymers with functional groups, which impart specific chemical reactivity or physical, chemical, structural, biological, and pharmacological functionality. The scope covers organic polymers, acting for instance as reagents, catalysts, templates, ion-exchangers, selective sorbents, chelating or antimicrobial agents, drug carriers, sensors, membranes, and hydrogels. This also includes reactive cross-linkable prepolymers and high-performance thermosetting polymers, natural or degradable polymers, conducting polymers, and porous polymers.
Original research articles must contain thorough molecular and material characterization data on synthesis of the above polymers in combination with their applications. Applications include but are not limited to catalysis, water or effluent treatment, separations and recovery, electronics and information storage, energy conversion, encapsulation, or adhesion.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


