通过多元醇还原工艺合成的磁弹道核壳结构 Ag@Fe3O4 粒子具有细菌消融和染料降解双重功能
IF 5.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
纳米银粒子具有强大的除菌能力,但在水溶液中容易聚集,影响抗菌效果。此外,污水处理中使用的银纳米粒子难以回收利用,造成环境污染和资源浪费。本文通过一步多元醇还原工艺,利用 Ag+/Ag0 和 Fe3+/Fe2+ 之间的还原电位差合成了 Ag 核 Fe3O4 壳结构颗粒(Ag@Fe3O4)。Ag@Fe3O4 复合材料中的 Fe3O4 外壳不仅能有效抑制 Ag 的团聚,还能增强复合材料对生物膜的渗透能力,从而使 Ag@Fe3O4 对大肠杆菌(E. coli)具有显著的抗菌功效。在 0.24 mg mL-1 的浓度下(Ag 含量为 0.042 mg mL-1),Ag@Fe3O4 对大肠杆菌的抑制率接近 100%,即使重复使用 10 个周期后,抗菌效果仍能保持 74.6%。同时,由于 Ag 具有优异的电子传导性和 Fe3O4 外壳对有机染料的有效吸附能力,Ag@Fe3O4 可促进电子快速转移到有机染料,并在 NaBH4 的存在下进一步导致有机染料的还原和降解。Ag@Fe3O4 可在短短 15 分钟内催化降解多种有机染料(包括罗丹明 B、罗丹明 6G 和亚甲蓝),经过 6 次循环再利用后,降解效率超过 90.9%。Ag@Fe3O4的成本效益(约为每克0.17美元)、便捷的磁性回收以及卓越的抗菌和染料降解性能,展示了其在医疗应用和污水处理方面的巨大潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
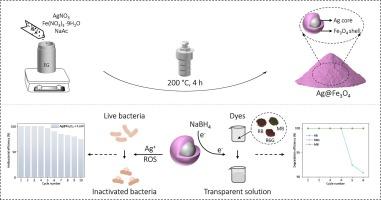
Magnetoplasmonic core–shell structured Ag@Fe3O4 particles synthesized via polyol reduction process rendering dual-functionality for bacteria ablation and dyes degradation
The Ag nanoparticles demonstrate potent bacteria eradication capabilities; however, their tendency to aggregate in aqueous solutions compromises the antibacterial efficacy. Furthermore, the Ag nanoparticles employed in sewage treatment are challenging to recycle, resulting in environmental pollution and resource wastage. Herein, the Ag-core Fe3O4-shell structured particles (Ag@Fe3O4) are synthesized by leveraging the reduction potential difference between Ag+/Ag0 and Fe3+/Fe2+ through a one-step polyol reduction process. The Fe3O4 shell in the Ag@Fe3O4 composite not only effectively inhibits the agglomeration of Ag, but also enhances the penetration capability of the composite into biofilms, thereby enabling Ag@Fe3O4 to possess remarkable antibacterial efficacy against Escherichia coli (E. coli). The Ag@Fe3O4 demonstrates nearly 100 % inhibition of E. coli at a concentration of 0.24 mg mL−1 (with an Ag content of 0.042 mg mL−1) while still maintaining antibacterial effectiveness of 74.6 % even after undergoing reutilization for 10 cycles. Meanwhile, due to the excellent electron conductivity of Ag and the effective adsorption capability of Fe3O4 shell towards organic dyes, Ag@Fe3O4 facilitates rapid electron transfer to organic dyes and further lead to their reduction and degradation in the presence of NaBH4. The Ag@Fe3O4 can catalytically degrade various organic dyes (including Rhodamine B, Rhodamine 6G, and Methylene blue) within only 15 min, while achieving an impressive degradation efficiency exceeding 90.9 % after 6 cycles of reutilization. The cost-effectiveness (approximately $0.17 per gram), facile magnetic recovery, along with the superior antibacterial and dye-degradation performance showcase the significant potential of Ag@Fe3O4 for medical applications and sewage treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Arabian Journal of Chemistry
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
10.80
自引率
3.30%
发文量
763
审稿时长
63 days
期刊介绍:
The Arabian Journal of Chemistry is an English language, peer-reviewed scholarly publication in the area of chemistry. The Arabian Journal of Chemistry publishes original papers, reviews and short reports on, but not limited to: inorganic, physical, organic, analytical and biochemistry.
The Arabian Journal of Chemistry is issued by the Arab Union of Chemists and is published by King Saud University together with the Saudi Chemical Society in collaboration with Elsevier and is edited by an international group of eminent researchers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


