双磷酸盐壳层结构装饰的 K0.45Rb0.05Mn0.85Mg0.15O2 阴极,用于提高钾/钠储量
IF 8.1
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
新型 K0.45Rb0.05Mn0.85Mg0.15O2(KRMMO)阴极由双磷酸盐 K3PO4/Mn3PO4 壳层封装,设计精巧,可提高钾/钠储量。得益于双磷酸盐层有效抑制了 KRMMO 的体积膨胀,K3PO4/MnPO4 双涂层 K0.45Rb0.05Mn0.85Mg0.15O2 (D-KRMMO)具有高电子传导性和快速离子扩散性,可促进钾/钠储存。同时,双磷酸盐 K3PO4/Mn3PO4 外壳直接将阴极与电解质隔离,减轻了电解质与材料之间发生的副反应,从而在循环过程中抑制了 KRMMO 主体中锰的溶解。在钾离子电池(PIBs)中,与纯 KRMMO(在 20 mA g-1 电流密度下为 94 mAh g-1)相比,D-KRMMO 在 20 mA g-1 电流密度下的放电比容量为 105.9 mAh g-1。在钠离子电池(SIBs)中,100 mA g-1 时的高可逆放电比容量为 112.8 mAh g-1。在 100 mA g-1 和 2 A g-1 条件下,C 率分别为 112.8 mAh g-1 和 44.8 mAh g-1。EIS 和 GITT 测量结果表明,D-KRMMO 阴极具有更快的离子迁移率和电子迁移率,以及更高的假电容贡献率。本文章由计算机程序翻译,如有差异,请以英文原文为准。
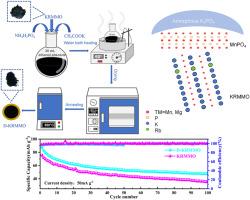
Bisphosphate shell layer structure-decorated K0.45Rb0.05Mn0.85Mg0.15O2 cathode for boosting potassium/sodium storage
Novel K0.45Rb0.05Mn0.85Mg0.15O2 (KRMMO) cathode encapsulated by bisphosphate K3PO4/Mn3PO4 shell layer is delicately designed for boosting potassium/sodium storage. Benefiting from the bisphosphate layer, the volume expansion of KRMMO is effectively inhibited, K3PO4/MnPO4 double-coated K0.45Rb0.05Mn0.85Mg0.15O2 (D-KRMMO) has a high electronic conductivity and fast ionic diffusivity, which can stimulate potassium/sodium storage. Meanwhile, bisphosphate K3PO4/Mn3PO4 shell directly isolates the cathode from the electrolyte to alleviate side reactions occurring between the electrolyte and the material, thus the dissolution of Mn in KRMMO host can be inhibited during the cycling process. In potassium ion batteries (PIBs), the discharge specific capacity of D-KRMMO is 105.9 mAh g−1 at a current density of 20 mA g−1 compared with pure KRMMO (94 mAh g−1 at 20 mA g−1). A highly reversible discharge specific capacity of 112.8 mAh g−1 at 100 mA g−1 is shown in the sodium-ion batteries (SIBs). And acceptable C-rate performances of 112.8 mAh g−1 and 44.8 mAh g−1 are exhibited at 100 mA g−1 and 2 A g−1, respectively. EIS and GITT measurements have shown that D-KRMMO cathode has faster ion mobility and electron mobility, as well as higher pseudocapacitance contribution.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


