化学计量辅助分光光度法同时测定幽门螺旋杆菌新治疗方案中的药物 - 环境可持续性评估
IF 5.5
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
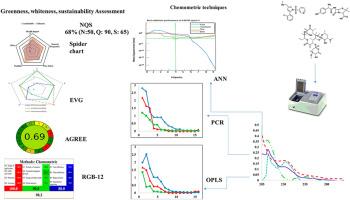
美国食品及药物管理局最近批准了 VOQUEZNA™ TRIPLE PAK™ 7 天疗法,其中包括 VON、阿莫西林 (AMO) 和克拉霉素 (CLA),这标志着幽门螺杆菌(H. pylori)感染治疗取得了重大进展。这些活性药物成分 (API) 的精确定量对于确保疗效和安全性至关重要。然而,传统的分析方法往往需要对样品进行大量的预处理和分离,既耗时又对环境造成负担。本研究首次采用符合绿色分析化学(GAC)原则的创新型化学计量辅助分光光度法对 VON、AMO 和 CLA 进行了同时定量。我们的方法无需预处理或分离,从而提高了分析效率和环境可持续性。我们开发了正交偏最小二乘法 (OPLS)、主成分回归法 (PCR) 和人工神经网络 (ANN) 模型,利用实验设计 (DoE) 方法最大限度地减少溶剂的使用和浪费。模型的精度很高,回收率在 98.00% 到 102.00% 之间。所有模型的 R2 值和 Q2 值均接近 1.0,表明定标样本具有很高的解释和预测能力,定标均方根误差(RMSEC)值小于 0.1。采用预测均方根误差(RMSEP)和预测相对均方根误差(RRMSEP)对验证集进行预测,发现 RMSEP 和 RRMSEP 的值分别为(0.0335-0.0613)和(0.7207-0.5287),而偏差校正后的预测均方根误差(BCMSEP)介于 0.0014 和 0.0001 之间。为了评估和提高这些方法的可持续性,使用了一些综合工具:SPIDER 溶剂工具、RGB12 算法、AGREE 和需求质量可持续性 (NQS) 指数。这项工作展示了环境可持续分析方法的进步,有助于实现可持续发展目标(SDGs)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Chemometric-assisted spectrophotometric methods for simultaneous drug determination in new Helicobacter pylori treatment regimens - Environmental sustainability assessment
The recent FDA approval of VOQUEZNA™ TRIPLE PAK™ 7-day therapy, which includes vonoprazan (VON), amoxicillin (AMO), and clarithromycin (CLA), marks a significant advancement in the treatment of Helicobacter pylori (H. pylori) infections. Accurate quantification of these active pharmaceutical ingredients (APIs) is critical for ensuring therapeutic efficacy and safety. However, conventional analytical methods often require extensive sample pretreatment and separation, which can be time-consuming and environmentally burdensome. This study presents the first simultaneous quantification of VON, AMO, and CLA using innovative chemometric-assisted spectrophotometric methods aligned with Green Analytical Chemistry (GAC) principles. Our methods eliminate the need for pretreatment or separation, thereby enhancing both analytical efficiency and environmental sustainability. We developed orthogonal partial least squares (OPLS), principal component regression (PCR), and Artificial Neural Network (ANN) models, utilizing the Design of Experiment (DoE) approach to minimize solvent use and waste.
Model validation was achieved through Orthogonal Array-based Latin Hypercube Sampling (OALHS), ensuring robust performance evaluation. The models demonstrated high precision, with recovery percentages ranging from 98.00% to 102.00%. The calibration set model fitting was assessed using the determination coefficient (R2), and the cross-validation coefficient (Q2), all model's R2 and Q2 values were close to 1.0, indicating the calibration samples' high capacity for explanation and prediction, while the root mean square error of calibration (RMSEC) values were found to be less than 0.1. The prediction of the validation set was employed by the root mean square error of prediction (RMSEP) and relative root mean square errors of prediction (RRMSEP), the values were found (0.0335–0.0613) and (0.7207–0.5287) for RMSEP and RRMSEP, respectively, while the bias-corrected mean square error of prediction (BCMSEP) was found to be between 0.0014 and 0.0001. To evaluate and enhance the sustainability of the methods, comprehensive tools were utilized: SPIDER Solvent Tool, RGB12 Algorithm, AGREE, and the Need Quality Sustainability (NQS) Index. This work supports the Sustainable Development Goals (SDGs) by demonstrating advancements in environmentally sustainable analytical methods.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Sustainable Chemistry and Pharmacy
Environmental Science-Pollution
CiteScore
8.20
自引率
6.70%
发文量
274
审稿时长
37 days
期刊介绍:
Sustainable Chemistry and Pharmacy publishes research that is related to chemistry, pharmacy and sustainability science in a forward oriented manner. It provides a unique forum for the publication of innovative research on the intersection and overlap of chemistry and pharmacy on the one hand and sustainability on the other hand. This includes contributions related to increasing sustainability of chemistry and pharmaceutical science and industries itself as well as their products in relation to the contribution of these to sustainability itself. As an interdisciplinary and transdisciplinary journal it addresses all sustainability related issues along the life cycle of chemical and pharmaceutical products form resource related topics until the end of life of products. This includes not only natural science based approaches and issues but also from humanities, social science and economics as far as they are dealing with sustainability related to chemistry and pharmacy. Sustainable Chemistry and Pharmacy aims at bridging between disciplines as well as developing and developed countries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


