通过 ReaxFF 分子动力学模拟研究甲烷燃料与二甲醚/氢/氨混合后的氧化机理
IF 6.7
1区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
摘要
用于微型发电系统的碳氢化合物燃料具有能量密度高、重量轻、供电时间长等显著优势,因此受到广泛关注。甲烷因其出色的可燃性、易于制备和方便储存而尤其受到青睐。本研究采用 ReaxFF 分子动力学模拟研究了三种添加剂(二甲醚、氢气和氨)对甲烷燃料燃烧机理的影响。结果表明,CH4 的燃烧途径主要涉及脱氢和氧化反应。在 CH4/C2H6O 系统中,二甲醚主要通过两种途径消耗:C-O 裂解和脱氢。这些途径会形成大量活性自由基(如 CH3 和 CH3O)。在 CH4/H2 燃烧中,氢被消耗,通过 H2 + OH → H2O + H、H2 + O2 → 2OH 和 H2 + 2O2 → 2HO2 等反应提供 H、OH 和 HO2 活性自由基。在燃烧 CH4/NH3 的过程中,氨最初会发生脱氢反应,涉及 OH、O 和 H 自由基,生成 NH2。随后,65.57% 的 NH2 进一步反应生成 H2O2N,16.39% 的 NH2 形成 NH 自由基。添加剂的存在会影响不同体系中的最终产物。在 CH4/C2H6O 系统中,最终产物包括 CO、CO2、H2 和 H2O。在 CH4/H2 系统中,最终产物包括 CO、CO2 和 H2O。最后,在 CH4/NH3 系统中,最终产物包括 CO、CO2、H2、NO、NO2 和 H2O。本文章由计算机程序翻译,如有差异,请以英文原文为准。
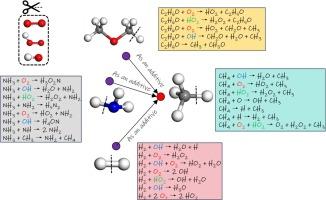
Oxidation mechanism of methane fuel blended with dimethyl ether/hydrogen/ammonia via ReaxFF molecular dynamics simulation
Hydrocarbon fuels used in micropower systems offer significant advantages, such as high energy density, lightweight properties, and extended power supply duration, making them the focus of widespread interest. Methane is particularly favored due to its excellent combustibility, ease of preparation, and convenient storage. In this study, ReaxFF molecular dynamics simulation is employed to investigate the influence of three additives (dimethyl ether, hydrogen, and ammonia) on the combustion mechanism of methane fuel. Results show that the pathways of CH4 mainly involve dehydrogenation and oxidation reactions. In CH4/C2H6O system, dimethyl ether is predominantly consumed via two pathways: C–O cleavage and dehydrogenation. These pathways form a significant quantity of active free radicals (such as CH3 and CH3O). In the case of CH4/H2 combustion, hydrogen is consumed to provide H, OH, and HO2 active free radicals via reactions such as H2 + OH → H2O + H, H2 + O2 → 2OH, and H2 + 2O2 → 2HO2. In the combustion of CH4/NH3, ammonia initially undergoes a dehydrogenation reaction involving OH, O, and H free radicals, resulting in the formation of NH2. Subsequently, 65.57 % of NH2 undergoes further reactions to form H2O2N, while 16.39 % of NH2 forms NH radicals. The presence of additives influences the final products in different systems. In the CH4/C2H6O system, the final products include CO, CO2, H2, and H2O. In the CH4/H2 system, the final products consist of CO, CO2, and H2O. Lastly, in the CH4/NH3 system, the final products comprise CO, CO2, H2, NO, NO2, and H2O.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fuel
工程技术-工程:化工
CiteScore
12.80
自引率
20.30%
发文量
3506
审稿时长
64 days
期刊介绍:
The exploration of energy sources remains a critical matter of study. For the past nine decades, fuel has consistently held the forefront in primary research efforts within the field of energy science. This area of investigation encompasses a wide range of subjects, with a particular emphasis on emerging concerns like environmental factors and pollution.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


