利用线形天冬水提取物在体外调节肠上皮细胞的抗炎和屏障保护作用
Q3 Pharmacology, Toxicology and Pharmaceutics
引用次数: 0
摘要
背景路依保斯是一种天然草本植物,含有多种独特的多酚,这些多酚与某些健康益处有关。本研究旨在比较未经发酵和发酵的路依保斯水提取物对猪肠上皮细胞(IPEC-J2)的抗炎和屏障保护作用。方法制备未经发酵和发酵的路依保斯水提取物,并对每种提取物进行化学和抗氧化分析。用未发酵或发酵的路依保斯水提取物(0.1 和 0.05 毫克/毫升)预处理猪肠上皮细胞(IPEC-J2),然后将其暴露于 10 微克/毫升的大肠杆菌脂多糖(LPS)中。对细胞活力、促炎细胞因子的蛋白质和基因表达进行调查,以确定两种提取物的抗炎效果。通过测量跨上皮电阻(TEER)和量化紧密连接基因的表达,研究了 IPEC-J2 的屏障完整性。有趣的是,对于某些基因(IL-8、IL-6、IL-1B 和 CCL20),发酵提取物,尤其是 0.1 mg/ml 的发酵提取物,在 LPS 诱导的炎症后能更有效地降低其表达,而对于另一种基因(TNF-α),两种浓度的未发酵提取物都能更有效地降低其表达,并最终保护 IPEC-J2 细胞免受炎症侵袭。在评估屏障完整性时,0.1 毫克/毫升的未发酵罗汉果提取物通过上调紧密连接蛋白、ZO-1、Occludin 和 Claudin-4 显示出最显著的屏障保护作用。这归因于每种萃取物的多酚含量不同,并证明了不同的路依保斯萃取物在减少早期炎症的发生方面具有独特的活性机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
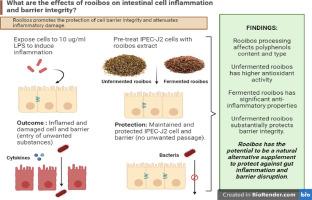
Modulating the anti-inflammatory and barrier protective effects on intestinal epithelial cells utilizing an aqueous Aspalathus linearis extract in vitro
Background
Rooibos is a natural herbal plant containing numerous unique polyphenols which have been associated with certain health benefits. Several of these bioactive constituents have been linked to promoting gut health through its beneficial actions such as anti-inflammatory and anti-spasmodic characteristics.
Purpose
The study aims to compare the anti-inflammatory and barrier protective effects of an unfermented and fermented rooibos aqueous extract on intestinal porcine epithelial cells (IPEC-J2).
Methods
Aqueous extracts of unfermented and fermented rooibos were prepared, and chemical and antioxidant analysis were performed of each extract. Intestinal porcine epithelial cells (IPEC-J2) were pre-treated with either unfermented or fermented aqueous rooibos extracts (0.1 and 0.05 mg/ml) then exposed to 10 µg/ml of Escherichia coli lipopolysaccharide (LPS). Cell viability, protein and gene expression of pro-inflammatory cytokines were investigated to determine the anti-inflammatory effect of both extracts. Barrier integrity of IPEC-J2 was investigated by measuring the Transepithelial electrical resistance (TEER) and quantifying tight junction gene expression.
Results
Both the unfermented and fermented rooibos extracts have significant anti-inflammatory properties by reducing pro-inflammatory cytokine production. Interestingly for certain genes (IL-8, IL-6, IL-1B and CCL20) the fermented extracts, specifically at 0.1 mg/ml, exhibited greater effectiveness by decreasing the expression after LPS-induced inflammation whilst for another gene (TNF-α) the unfermented extract at both concentrations was more efficient in decreasing expression and ultimately protecting the IPEC-J2 cells from inflammation. When evaluating the barrier integrity, 0.1 mg/ml of the unfermented rooibos extract showed the most significant barrier protective effects through upregulation of tight junction proteins, ZO-1, Occludin as well as Claudin-4.
Conclusion
Higher concentration of the fermented rooibos extract prevented the inflammatory response significantly whilst the higher concentration of the unfermented rooibos extract enhanced tight junction expression and protected barrier integrity in LPS-induced inflamed intestinal cells. This is attributed to the differences in the polyphenol content of each extract and demonstrating the unique activity mechanisms of different rooibos extracts to reduce onset of early inflammation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytomedicine Plus
Medicine-Complementary and Alternative Medicine
CiteScore
3.70
自引率
0.00%
发文量
178
审稿时长
81 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


