揭示塞马鲁肽对糖尿病大鼠睾丸功能障碍模型的影响:对精子发生途径和 miRNA-148a-5p 的见解。
IF 2.3
4区 医学
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
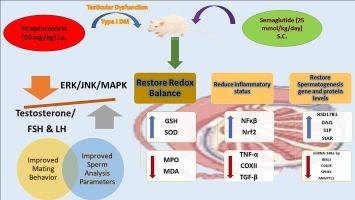
背景:长期以来,糖尿病一直是导致男性性功能障碍的一个众所周知的危险因素,其原因可能是持续的高血糖、氧化应激和精子发生抑制。本研究探讨了塞马鲁肽(Sem)缓解糖尿病大鼠睾丸功能障碍和精子生成障碍的潜力,以了解这种保护作用的分子机制:方法:进行对照实验,将28只成年雄性大鼠分为四组:对照组、塞马鲁肽组、糖尿病组和糖尿病+塞马鲁肽组。对照组使用单剂量 STZ(50 毫克/千克,静脉注射)诱导糖尿病。同时,在确诊糖尿病后,每天皮下注射塞马鲁肽(25 nmol/kg),持续四周。除了交配行为评估外,还进行了多项生化和组织化学分析。结果表明,Sem治疗可有效缓解糖尿病引起的性功能和睾丸功能障碍。具体来说,它能调节被破坏的氧化还原平衡,恢复精子发生基因和蛋白水平,调节激素谱,减轻睾丸炎:Sem通过影响多种途径和恢复精子发生相关基因和蛋白,防止糖尿病引起的睾丸和性功能损伤。未来的研究可能会涉及对Sem在临床中的转化应用进行潜在调查,以治疗与糖尿病相关的男性不育症。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Unraveling the impact of semaglutide in a diabetic rat model of testicular dysfunction: Insights into spermatogenesis pathways and miRNA-148a-5p
Background
Diabetes has been a long-known risk factor for male sexual dysfunction, which may be caused by persistent hyperglycemia, oxidative stress, and spermatogenesis inhibition. This study explored the potential of Semaglutide (Sem) to alleviate testicular dysfunction and spermatogenesis impairment in diabetic rats to understand the molecular mechanism of this protective effect.
Methodology
A controlled experiment was conducted where 28 adult male rats were divided into four groups: control, Semaglutide, diabetic, and diabetes + Sem. Diabetes was induced using a single STZ dose (50 mg/kg, i.p.). At the same time, Sem was administered as a daily subcutaneous dose (25 nmol/kg) for four weeks after the confirmed diagnosis of diabetes.
Several biochemical and histochemical analyses were performed in addition to mating behavior assessments. The estimation of spermatogenesis-related genes and proteins was conducted using PCR and western blotting techniques.
Results
revealed promising outcomes, wherein Sem treatment effectively mitigated diabetes-induced sexual and testicular dysfunction. Specifically, it regulated the disrupted redox balance, restored spermatogenesis gene and protein levels, modulated hormonal profiles, and mitigated testicular inflammation.
Conclusion
Sem protects against diabetes-induced testicular and sexual impairments by influencing several pathways and restoring spermatogenesis-related genes and proteins. Future studies may involve a potential investigation of Sem translational applications in clinical settings for treating male infertility associated with diabetes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Steroids
医学-内分泌学与代谢
CiteScore
5.10
自引率
3.70%
发文量
120
审稿时长
73 days
期刊介绍:
STEROIDS is an international research journal devoted to studies on all chemical and biological aspects of steroidal moieties. The journal focuses on both experimental and theoretical studies on the biology, chemistry, biosynthesis, metabolism, molecular biology, physiology and pharmacology of steroids and other molecules that target or regulate steroid receptors. Manuscripts presenting clinical research related to steroids, steroid drug development, comparative endocrinology of steroid hormones, investigations on the mechanism of steroid action and steroid chemistry are all appropriate for submission for peer review. STEROIDS publishes both original research and timely reviews. For details concerning the preparation of manuscripts see Instructions to Authors, which is published in each issue of the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


