四苓煎剂通过AKT/IKKβ/NFκB信号通路改善腺嘌呤诱导的大鼠肾脏纤维化。
IF 6.7
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
目的通过网络药理学分析和实验验证,探讨四苓汤(SLD)如何减轻慢性肾脏病(CKD)大鼠的肾脏纤维化:方法:通过中药数据库(TCMSP)和液相色谱质谱法(LC-MS)初步确定了四苓汤(伏苓、竹苓、泽泻、白术)的主要活性成分及其靶向作用。通过 GeneCard、OMIM、PharmGkb 和 GEO 等数据库筛选出肾脏纤维化的治疗靶点。随后,构建了药物-疾病-靶点网络,并进行了PPI分析。对相互交叉的靶点进行了 GO 和 KEGG 通路富集分析。通过腺嘌呤灌胃诱导肾脏纤维化模型,然后用SLD治疗。采用Masson、天狼星红、免疫组化和Western blot等方法检测肾功能和纤维化相关指标。该机制在体外实验中得到了进一步验证:结果:网络药理学分析发现了100个与SLD活性成分相关的常见靶点,包括AKT1和CCND1等核心基因。GO富集分析显示,受影响最大的三个生物过程包括对异生物刺激的反应、对营养水平的反应和对氧化应激的反应。这些过程涉及膜筏、膜微域和突触膜等细胞成分,其分子功能主要与泛素样蛋白连接酶结合、泛素蛋白连接酶结合、DNA结合转录因子结合和RNA聚合酶II特异性DNA结合转录因子结合有关。KEGG通路富集分析表明,脂质与动脉粥样硬化、PI3K-AKT信号通路和前列腺癌等通路可能参与了SLD的抗纤维化作用。值得注意的是,AKT/IKKβ/NFκB 信号通路是一个关键机制。这些发现在体内和体外得到了进一步证实:结论:SLD能有效改善腺嘌呤诱导的大鼠慢性肾脏病纤维化,可能是通过抑制AKT/IKKβ/NFκB信号通路实现的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
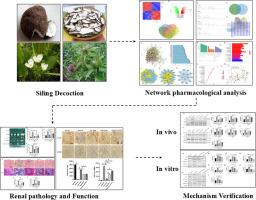
Siling decoction ameliorates adenine-induced renal fibrosis in rats by the AKT/IKKβ/NFκB signaling pathway
Objective
Investigating how Siling decoction (SLD) mitigates fibrosis in rats with chronic kidney disease CKD (chronic kidney disease) through network pharmacology analysis and experimental verification.
Methods
Initially, the primary active components and their target actions of SLD (Fuling, Zhuling, Zexie, and Baizhu) were identified by the TCMSP database and liquid chromatography mass spectrometry (LC-MS). Treatment targets for renal fibrosis were screened through databases such as GeneCard, OMIM, PharmGkb, and GEO. Subsequently, a drug-disease-target network was constructed and subjected to PPI analysis. Intersecting targets underwent GO and KEGG pathway enrichment analyses. Renal fibrosis model was induced by adenine gavage, then treat with SLD. Masson, Sirius red, immunohistochemistry, and Western blot were used to detect renal function and fibrosis-related indicators. The mechanism was further validated in vitro experiments.
Results
Network pharmacology analysis identified 100 common targets associated with the active components of SLD, including core genes such as AKT1 and CCND1. GO enrichment analysis revealed that the top three biological processes impacted include response to xenobiotic stimulus, response to nutrient levels and response to oxidative stress. These processes involved cellular components such as membrane raft, membrane microdomain and synaptic membrane, with molecular functions predominantly associated with ubiquitin-like protein ligase binding, ubiquitin protein ligase binding, DNA-binding transcription factor binding, and RNA polymerase II-specific DNA binding transcription factor binding. KEGG pathway enrichment analysis indicated potential involvement of pathways like Lipid and atherosclerosis, PI3K-AKT signaling pathway, and prostate cancer are likely involved in the anti-fibrotic effect of SLD. Notably, the highlighted was the AKT/IKKβ/NFκB signaling pathway as a key mechanism. These findings were further confirmed in vivo and in vitro.
Conclusion
The SLD effectively ameliorates adenine-induced chronic kidney disease fibrosis in rats, potentially by inhibiting the AKT/IKKβ/NFκB signaling pathway.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


