评估基于吡啶氮冠化合物 H4PATA、PATAM 和 H4PATPA 的螯合剂对 68Ga 和 177Lu 的作用。
IF 3.6
4区 医学
Q1 RADIOLOGY, NUCLEAR MEDICINE & MEDICAL IMAGING
引用次数: 0
摘要
本文介绍了基于吡啶氮冠化合物的三种大环螯合剂 H4PATA、PATAM 和 H4PATPA 的合成和表征。我们利用 MALDI TOF MS、1H NMR 光谱、放射性标记研究以及胎牛血清和摩尔过量 1000 倍 H4EDTA 的体外实验对它们与 68Ga 和 177Lu 的复合物进行了深入研究。我们的研究表明,螯合剂 H4PATA 和 H4PATPA 可在室温下与两种放射性核素形成复合物(30 分钟后,与 H4PATA 和 H4PATPA 复合物的 RCY 分别大于 80% 和大于 90%)。螯合剂 PATAM 需要高温(95 °C)进行络合。在胎牛血清中进行的体外稳定性检测以及 H4EDTA 挑战表明,所有与 68Ga 的络合物都会发生转切作用。然而,配体 H4PATA 和 PATAM 与 177Lu 的络合物却很稳定。因此,考虑到[177Lu]Lu-PATA 复合物在室温下的放射性标记和体外稳定性,我们的研究表明螯合剂 H4PATA 是 177Lu 放射性药物的候选物质。本文章由计算机程序翻译,如有差异,请以英文原文为准。
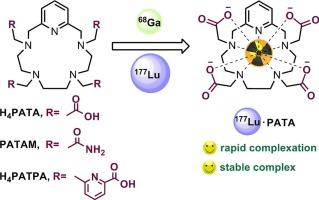
Evaluation of chelating agents based on pyridine-azacrown compounds H4PATA, PATAM, and H4PATPA for 68Ga and 177Lu
In this article, we present the synthesis and characterization of three macrocyclic chelators, H4PATA, PATAM, and H4PATPA, based on a pyridine-azacrown compound. Their complexation with 68Ga and 177Lu has been thoroughly investigated using MALDI TOF MS, 1H NMR spectroscopy, radiolabeling studies, and experiments in vitro with fetal bovine serum and a 1000-fold molar excess of H4EDTA. Our studies have shown that the chelators H4PATA and H4PATPA form complexes at room temperature with both radionuclides (RCY > 80 % and > 90 % for complexes with H4PATA and H4PATPA after 30 min, respectively). The chelator PATAM requires high temperature (95 °C) for complexation. In vitro stability assays in fetal bovine serum as well as H4EDTA-challenge revealed that transchelation occurs for all complexes with 68Ga. However, complexes of the ligands H4PATA and PATAM with 177Lu were found stable. Thus, taking into account the radiolabeling at room temperature and in vitro stability of the complex [177Lu]Lu·PATA, our investigations revealed the chelator H4PATA is a candidate for radiopharmaceutical use with 177Lu.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nuclear medicine and biology
医学-核医学
CiteScore
6.00
自引率
9.70%
发文量
479
审稿时长
51 days
期刊介绍:
Nuclear Medicine and Biology publishes original research addressing all aspects of radiopharmaceutical science: synthesis, in vitro and ex vivo studies, in vivo biodistribution by dissection or imaging, radiopharmacology, radiopharmacy, and translational clinical studies of new targeted radiotracers. The importance of the target to an unmet clinical need should be the first consideration. If the synthesis of a new radiopharmaceutical is submitted without in vitro or in vivo data, then the uniqueness of the chemistry must be emphasized.
These multidisciplinary studies should validate the mechanism of localization whether the probe is based on binding to a receptor, enzyme, tumor antigen, or another well-defined target. The studies should be aimed at evaluating how the chemical and radiopharmaceutical properties affect pharmacokinetics, pharmacodynamics, or therapeutic efficacy. Ideally, the study would address the sensitivity of the probe to changes in disease or treatment, although studies validating mechanism alone are acceptable. Radiopharmacy practice, addressing the issues of preparation, automation, quality control, dispensing, and regulations applicable to qualification and administration of radiopharmaceuticals to humans, is an important aspect of the developmental process, but only if the study has a significant impact on the field.
Contributions on the subject of therapeutic radiopharmaceuticals also are appropriate provided that the specificity of labeled compound localization and therapeutic effect have been addressed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


