α-乳白蛋白颗粒在生理相关条件下的分解和体外细胞相容性。
IF 4.3
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
有序超分子结构形式的蛋白质自组装(如微粒)具有作为新型生物材料的巨大潜力。然而,该领域的研究很少在生理相关条件下进行,但要揭示微粒和其他淀粉样结构在健康科学中的潜在用途,此类研究是非常必要的。本研究通过热孵育法制备了处于不同成熟阶段的α-乳白蛋白(ALA)微粒。通过同时测量硫黄素 T 荧光强度和光散射,研究了微粒在等渗缓冲液、中性 pH 和 37 °C条件下的分解情况。在体外评估了不同成熟阶段的微粒对肺上皮细胞和成纤维细胞的生物相容性。在微粒及其释放物存在的情况下,观察到了良好的细胞相容性。我们的研究结果表明,蛋白质微粒具有生物可降解性和高度可调性,是药物输送的理想候选材料。本文章由计算机程序翻译,如有差异,请以英文原文为准。
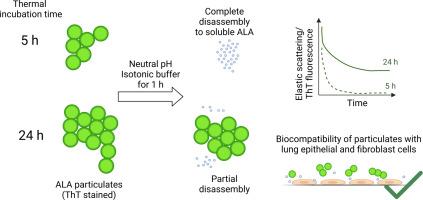
Disassembly and in vitro cell compatibility of α-lactalbumin particulates under physiologically relevant conditions
Protein self-assemblies in the form of ordered supramolecular structures such as particulates hold great potential as new biomaterials. However, research in this field is rarely conducted under physiologically relevant conditions but such studies are crucially needed to unravel the potential use of particulates and other amyloid structures in health sciences. In this study, particulates of α-lactalbumin (ALA) were prepared at different stages of maturation by thermal incubation. Disassembly of particulates in isotonic buffer, neutral pH and at 37 °C was investigated by simultaneously measuring Thioflavin T fluorescence intensity and light scattering. Freshly formed particulates quickly disassembled and displayed complete release of soluble ALA within 1 h. Mature particulates displayed slower disassembly kinetics with incomplete release of ALA within 1 h. The biocompatibility of particulates at different maturation stages to epithelial lung and fibroblast cells was assessed in vitro. Good cell compatibility was observed in the presence of the particulates and their released species. Our findings display protein particulates as biodegradable and highly tunable particles, promoting them as good candidates for drug delivery purposes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


