通过透明质酸修饰的 ZIF-8 纳米粒子靶向输送赛可皂素 A 和多柔比星,用于 TNBC 治疗:抑制转移并降低心脏毒性
IF 5.5
2区 医学
Q2 MATERIALS SCIENCE, BIOMATERIALS
Materials Science & Engineering C-Materials for Biological Applications
Pub Date : 2024-11-12
DOI:10.1016/j.bioadv.2024.214114
引用次数: 0
摘要
三阴性乳腺癌(TNBC)是乳腺癌的一种侵袭性亚型,其特点是没有雌激素受体、孕激素受体和 HER2 表达,因此传统的激素和靶向治疗无效。化疗仍是 TNBC 的主要治疗方法,但它未能充分解决复发率和转移率高的问题,这凸显了对新治疗策略的迫切需求。本研究调查了一种从传统中药中提取的化合物--赛可皂甙 A(SSA),研究其在增强多柔比星(DOX)化疗疗效的同时减少 TNBC 转移和减轻 DOX 引起的心脏毒性的潜力。我们首先证实了 SSA 对 DOX 引起的心脏毒性具有保护作用,从而凸显了其作为 TNBC 化疗辅助疗法的潜力。随后,通过网络药理学分析,我们发现 SSA 可通过下调整合素 β3(肿瘤发生发展的关键调控因子)抑制 TNBC 的进展和转移。体内和体外实验进一步验证了这一点。为了解决 SSA 生物利用率低的问题,我们开发了一种新型给药系统,利用透明质酸(HA)修饰的沸石咪唑框架-8(ZIF-8)纳米颗粒共同给药 SSA 和 DOX。该纳米药物系统具有出色的稳定性和高载药能力,SSA和DOX的载药效率分别为40.07%和43.07%。给药 24 小时后,使用纳米给药系统组的 DOX 浓度是对照组的 5.01 倍,显示出更强的肿瘤靶向能力。此外,治疗 14 天后,肿瘤体积比对照组减少了 80.8%,表明疗效显著提高(所有 P本文章由计算机程序翻译,如有差异,请以英文原文为准。
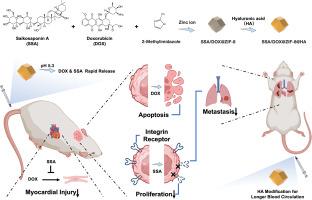
Targeted delivery of Saikosaponin A and doxorubicin via hyaluronic acid-modified ZIF-8 nanoparticles for TNBC treatment: Inhibiting metastasis and reducing cardiotoxicity
Triple-negative breast cancer (TNBC) is an aggressive subtype of breast cancer characterized by the absence of estrogen receptors, progesterone receptors, and HER2 expression, making traditional hormone and targeted therapies ineffective. Chemotherapy remains the primary treatment for TNBC; however, it has failed to adequately address the high rates of recurrence and metastasis, underscoring the urgent need for new therapeutic strategies. This study investigates Saikosaponin A (SSA), a compound extracted from traditional Chinese medicine, for its potential to enhance the efficacy of doxorubicin (DOX) chemotherapy while reducing TNBC metastasis and mitigating DOX-induced cardiotoxicity.
We first confirmed SSA's cardioprotective effects against DOX-induced cardiotoxicity, highlighting its potential as an adjunctive therapy for TNBC chemotherapy. Subsequently, through network pharmacology analysis, we identified that SSA may inhibit TNBC progression and metastasis by downregulating integrin β3, a key regulatory factor in tumor development. This was further validated through both in vivo and in vitro experiments. To address the poor bioavailability of SSA, we developed a novel drug delivery system utilizing hyaluronic acid (HA)-modified zeolitic imidazolate framework-8 (ZIF-8) nanoparticles for the co-delivery of SSA and DOX. This nano-drug system exhibited excellent stability and high drug-loading capacity, with loading efficiencies of 40.07 % for SSA and 43.07 % for DOX. After 24 h of nano-drug administration, the DOX concentration in the group using the nano-delivery system was 5.01 times higher than control group, demonstrated enhanced tumor-targeting capability. Furthermore, after 14 days of treatment, the tumor volume was reduced by 80.8 % compared to the control group, indicating significantly improved therapeutic efficacy (all P < 0.05).
This study systematically evaluates the potential of this dual drug-loaded nanocarrier in improving TNBC treatment, reducing DOX-induced cardiotoxicity, and inhibiting metastasis, offering a novel therapeutic approach that integrates traditional medicine with advanced nanotechnology.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
17.80
自引率
0.00%
发文量
501
审稿时长
27 days
期刊介绍:
Biomaterials Advances, previously known as Materials Science and Engineering: C-Materials for Biological Applications (P-ISSN: 0928-4931, E-ISSN: 1873-0191). Includes topics at the interface of the biomedical sciences and materials engineering. These topics include:
• Bioinspired and biomimetic materials for medical applications
• Materials of biological origin for medical applications
• Materials for "active" medical applications
• Self-assembling and self-healing materials for medical applications
• "Smart" (i.e., stimulus-response) materials for medical applications
• Ceramic, metallic, polymeric, and composite materials for medical applications
• Materials for in vivo sensing
• Materials for in vivo imaging
• Materials for delivery of pharmacologic agents and vaccines
• Novel approaches for characterizing and modeling materials for medical applications
Manuscripts on biological topics without a materials science component, or manuscripts on materials science without biological applications, will not be considered for publication in Materials Science and Engineering C. New submissions are first assessed for language, scope and originality (plagiarism check) and can be desk rejected before review if they need English language improvements, are out of scope or present excessive duplication with published sources.
Biomaterials Advances sits within Elsevier''s biomaterials science portfolio alongside Biomaterials, Materials Today Bio and Biomaterials and Biosystems. As part of the broader Materials Today family, Biomaterials Advances offers authors rigorous peer review, rapid decisions, and high visibility. We look forward to receiving your submissions!

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


