利用废弃锂离子电池中的改性石墨@壳聚糖协同处理含铍废水。
IF 7.7
1区 化学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
International Journal of Biological Macromolecules
Pub Date : 2024-11-15
DOI:10.1016/j.ijbiomac.2024.137698
引用次数: 0
摘要
为了回收并有效去除含铍废水中的铍,缓解废电池带来的环境压力。本研究以从废电池中分离出的改性石墨材料为基础,设计合成了石墨@壳聚糖复合凝胶(CWBG@CH)。有趣的是,在 pH = 6 和 35 °C 条件下,CWBG@CH 的最大拟合吸附容量(Qemax)为 83.54 mg/g。CWBG@CH 的吸附过程由表面络合和静电吸引控制。铍与 CWBG@CH 上的碳酸/羟基和磷酸/氨基之间的强配位和协同吸附增强了 CWBG@CH 对铍的吸附能力和选择性。同时,CWBG@CH 5 次吸附-解吸效率大于 85%。这一发现为从废电池中回收石墨材料提供了一个方向,并表明 CWBG@CH 在去除水溶液中的 Be(II) 方面具有巨大潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
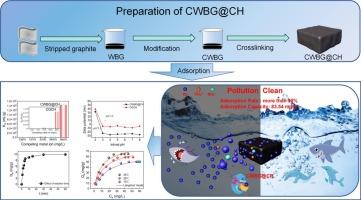
Collaborative disposal of beryllium−containing wastewater with modified graphite@chitosan from waste lithium−ion batteries
In order to recover and effectively remove beryllium from beryllium-containing wastewater and relieve the environmental pressure caused by waste batteries. In this study, the gel material was synthesized based on the modified graphite material separated from the waste battery, and the graphite−@chitosan composite gel (CWBG@CH) was designed and synthesized. Interestingly, CWBG@CH has a maximum fitted adsorption capacity (Qemax) of 83.54 mg/g at pH = 6 and 35 °C. The adsorption process of CWBG@CH is controlled by surface complexation and electrostatic attraction. Strong coordination and synergistic adsorption between Be and the carbonic acid/hydroxyl group and phosphoric acid/amino group on CWBG@CH enhances the adsorption capacity and selectivity of CWBG@CH for Be. At the same time, the adsorption-desorption efficiency of the CWBG@CH in 5 times is >85 %. This discovery provides a direction for the recycling of graphite materials from waste batteries and indicates the great potential of CWBG@CH to remove Be(II) from aqueous solutions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
13.70
自引率
9.80%
发文量
2728
审稿时长
64 days
期刊介绍:
The International Journal of Biological Macromolecules is a well-established international journal dedicated to research on the chemical and biological aspects of natural macromolecules. Focusing on proteins, macromolecular carbohydrates, glycoproteins, proteoglycans, lignins, biological poly-acids, and nucleic acids, the journal presents the latest findings in molecular structure, properties, biological activities, interactions, modifications, and functional properties. Papers must offer new and novel insights, encompassing related model systems, structural conformational studies, theoretical developments, and analytical techniques. Each paper is required to primarily focus on at least one named biological macromolecule, reflected in the title, abstract, and text.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


