将化学混合物的终生暴露维度纳入风险评估过程。微量元素的应用。
IF 3.5
3区 医学
Q2 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
摘要
一般人终生都会接触到各种化学品混合物。大多数情况下,风险评估是通过外部接触单一化学品并考虑一种接触途径来估算人群中产生不良影响的概率。为了估算某种化学品的总体暴露量,可使用人体生物监测研究来测量生物基质中的化学品浓度。这些研究的局限性在于无法区分接触源或接触途径。此外,由于相关成本的原因,通常只能测定有限几种化学品的浓度。在这项研究中,我们开发了一种方法来估算人口在一生中对痕量元素(无机砷、镉、铅和汞)混合物的体内暴露量。该方法采用现实的终生暴露轨迹,结合基于生理的动力学建模,考虑了多个暴露源。然后,将估计的生物标志物暴露与人类生物监测数据进行比较,以估计该方法的稳健性。最后,考虑到化学品的叠加效应,根据模拟的生物标志物暴露进行风险定性。这种方法可以确定化学品对肾脏效应总体风险的贡献。本文章由计算机程序翻译,如有差异,请以英文原文为准。
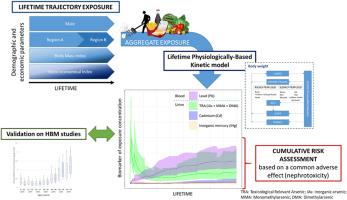
Integrating the lifelong exposure dimension of a chemical mixture into the risk assessment process. Application to trace elements
Lifelong, the general population is exposed to mixtures of chemicals. Most often, risk assessment is performed to estimate the probability of adverse effects in the population using external exposures to a single chemical and considering one route of exposure. To estimate whole exposure to a chemical, human biomonitoring studies are used to measure chemical concentrations in biological matrices. The limitations of these studies are that it is not possible to distinguish the sources or the routes of exposure. Moreover, only the concentrations of a limited number of chemicals are usually determined due to the associated cost. In this study, a methodology has been developed to estimate the internal exposures of the population to a mixture of trace elements (inorganic As, Cd, Pb and Hg) throughout lifetime. This methodology uses realistic lifetime exposure trajectories coupled to physiological based kinetic modeling, considering several sources of exposure. Then, the estimated biomarkers of exposure were compared to human biomonitoring data to estimate the robustness of the methodology. Finally, risk characterization was performed based on the simulated biomarkers of exposure considering an additive effect of chemicals. This methodology allows to determine the contribution of chemicals to the overall risk of renal effect.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food and Chemical Toxicology
工程技术-毒理学
CiteScore
10.90
自引率
4.70%
发文量
651
审稿时长
31 days
期刊介绍:
Food and Chemical Toxicology (FCT), an internationally renowned journal, that publishes original research articles and reviews on toxic effects, in animals and humans, of natural or synthetic chemicals occurring in the human environment with particular emphasis on food, drugs, and chemicals, including agricultural and industrial safety, and consumer product safety. Areas such as safety evaluation of novel foods and ingredients, biotechnologically-derived products, and nanomaterials are included in the scope of the journal. FCT also encourages submission of papers on inter-relationships between nutrition and toxicology and on in vitro techniques, particularly those fostering the 3 Rs.
The principal aim of the journal is to publish high impact, scholarly work and to serve as a multidisciplinary forum for research in toxicology. Papers submitted will be judged on the basis of scientific originality and contribution to the field, quality and subject matter. Studies should address at least one of the following:
-Adverse physiological/biochemical, or pathological changes induced by specific defined substances
-New techniques for assessing potential toxicity, including molecular biology
-Mechanisms underlying toxic phenomena
-Toxicological examinations of specific chemicals or consumer products, both those showing adverse effects and those demonstrating safety, that meet current standards of scientific acceptability.
Authors must clearly and briefly identify what novel toxic effect (s) or toxic mechanism (s) of the chemical are being reported and what their significance is in the abstract. Furthermore, sufficient doses should be included in order to provide information on NOAEL/LOAEL values.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


