N、P 共掺多孔生物炭支持缺电子的 Niδ+,用于利用甲酸甲酯将乙酰丙酸与硝基烯烃进行多米诺还原胺化和酰胺化,生成 N-取代的吡咯烷酮
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
从生物质衍生的左旋乙酸(LA)和硝基烯烃开始合成 N-取代的吡咯烷酮是一条极具吸引力的 LA 价值化途径,但仍然面临着挑战。在此,我们报告了一种缺电子的 Niδ+ 催化剂(Ni2P/NPCB-600),该催化剂是采用一锅碳化法在咖啡生物炭中掺入 N 和 P 制备而成的。与相应的金属镍催化剂(Ni/CB-600 和 Ni/NPCB-600)相比,Ni2P/NPCB-600 在 LA 与硝基烯烃的多米诺还原胺化和酰胺化反应中表现出更优异的催化性能。表征和对照实验表明,Ni2P/NPCB-600 的高催化活性和选择性主要归功于形成了缺电子的 Niδ+ (Ni2δ+-Pδ-),它可以促进甲酸甲酯的分解,并在多米诺反应过程中吸附和活化 LA 中的 C=O 键。此外,Ni2P/NPCB-600 还显示出良好的底物范围,可以直接从 LA 和不同的硝基烯烃合成各种 N-取代的吡咯烷酮,而且产率良好甚至极佳(87.5-99%)。这项研究为通过调整镍的电子密度高效生产 N-取代的吡咯烷酮提供了一条潜在的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
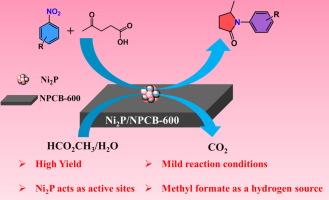
N, P co-doped porous biochar supported electron deficient Niδ+ for domino reductive amination and amidation of levulinic acid with nitroarenes to N-substituted pyrrolidones using methyl formate
The synthesis of N-substituted pyrrolidones starting from biomass-derived levulinic acid (LA) and nitroarenes is a highly attractive route for LA valorization, but still faces challenges. Herein, we report an electron deficient Niδ+ catalyst (Ni2P/NPCB-600) prepared by doping N and P into coffee biochar using a one-pot carbonization method. Ni2P/NPCB-600 shows outperform catalytic performance compared with the corresponding metallic Ni catalysts (Ni/CB-600 and Ni/NPCB-600) in the domino reductive amination and amidation of LA with nitroarenes. Characterization and controlled experiments demonstrate that the high catalytic activity and selectivity of Ni2P/NPCB-600 are mainly attributed to the formation of electron deficient Niδ+ (Ni2δ+-Pδ-), which could promote the decomposition of methyl formate, and the adsorption and activation of C=O bonds in LA during the domino reactions. Furthermore, Ni2P/NPCB-600 also shows good substrate scope, various N-substituted pyrrolidones can be directly synthesized from LA and different nitroarenes with good to excellent yields (87.5–99 %). This study provides a potential pathway for efficiently producing N-substituted pyrrolidones by adjusting the electron density of Ni.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


