电荷分离工程压电超薄纳米棒可调节肿瘤基质微环境并增强细胞免疫原性,从而实现压电-热-免疫协同疗法
IF 13
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
肿瘤微环境(TME)的特点是缺氧和低免疫原性,其致密而僵硬的细胞外基质(ECM)阻碍了治疗药物和免疫细胞的扩散,从而限制了免疫疗法的疗效。为了克服这些挑战,我们开发了一种氧缺陷压电光热敏化剂--钒酸铋纳米棒支撑铂纳米点(BVP)。铂的加入增强了光热效应,提高了超声波下的电荷分离效率,从而增加了热量的产生以及活性氧(ROS)和氧气的生成。铂还能催化 TME 中的过氧化氢转化为氧气,这既是 ROS 的来源,也是缓解肿瘤缺氧的手段,从而逆转具有免疫抑制作用的 TME。此外,铋离子与谷胱甘肽的配位作用进一步扩大了细胞氧化应激。产生的热量和 ROS 不仅会使 ECM 中的胶原蛋白变性,促进 BVP 深入肿瘤,还会诱导肿瘤细胞的免疫原性细胞死亡。通过 "变性和渗透 "策略,光声疗法可有效激活免疫细胞,抑制肿瘤生长和转移。这项研究开创性地提出了一种抗肿瘤纳米药物的设计方法,旨在逆转 TME 的免疫抑制特性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
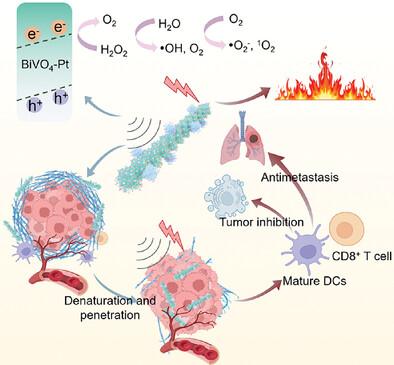
Charge Separation‐Engineered Piezoelectric Ultrathin Nanorods Modulate Tumor Stromal Microenvironment and Enhance Cell Immunogenicity for Synergistically Piezo‐Thermal‐Immune Therapy
The tumor microenvironment (TME) is characterized by hypoxia and low immunogenicity, with a dense and rigid extracellular matrix (ECM) that impedes the diffusion of therapeutic agents and immune cells, thereby limiting the efficacy of immunotherapy. To overcome these challenges, an oxygen defect piezoelectric‐photothermal sensitizer, bismuth vanadate nanorod‐supported platinum nanodots (BVP) is developed. The integration of platinum enhances the photothermal effect and improves charge separation efficiency under ultrasound, leading to increased heat generation and the production of reactive oxygen species (ROS) and oxygen. Platinum also catalyzes the conversion of hydrogen peroxide in the TME to oxygen, which serves as both a ROS source and a means to alleviate tumor hypoxia, thereby reversing the immunosuppressive TME. Moreover, the coordination of bismuth ions with glutathione further amplifies cellular oxidative stress. The generated heat and ROS not only denature the collagen in the ECM, facilitating the deeper penetration of BVP into the tumor but also induce immunogenic cell death in tumor cells. Through the “degeneration and penetration” strategy, photoacoustic therapy effectively activates immune cells, inhibiting both tumor growth and metastasis. This study introduces a pioneering approach in the design of antitumor nanomedicines aimed at reversing the immunosuppressive characteristics of the TME.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


