加强射频消融治疗肝细胞癌:纳米表皮药物对免疫调节和抗原性恢复的影响
IF 27.4
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
射频消融术(RFA)是治疗肝细胞癌(HCC)的一种重要疗法,但它有很大的复发和转移风险,特别是由于通过表观遗传修饰涉及免疫逃避和抗原下调的机制。本研究介绍了一种名为 MFMP 的 "纳米药物"。MFMP由中空介孔二氧化锰(MnO2)纳米颗粒、MAT2A抑制剂FIDAS-5、巨噬细胞膜和抗PD-L1(aPD-L1)组成,靶向HCC细胞。通过选择性地与这些细胞结合,MFMP 最初可通过抑制 PD-L1 逆转免疫抑制。内吞后,MFMP 在肿瘤微环境中分解,释放出 FIDAS-5 和 Mn2+。FIDAS-5 可防止 cGAS 甲基化,而 Mn2+ 则有助于 STING 通路的恢复。此外,FIDAS-5 还能减少 m6A RNA 修饰,抑制表皮生长因子受体的表达。这些变化增强了 HCC 的抗原性,从而促进细胞毒性 T 细胞的识别和细胞毒性杀伤。此外,MFMP 通过 cGAS DNA 去甲基化、表皮生长因子受体 mRNA 去甲基化和 TBK1 蛋白磷酸化与 RFA 协同作用,介导 HCC 免疫性细胞死亡,从而抑制复发和转移并增强免疫记忆。因此,MFMP 是一种潜在的辅助疗法,需要临床验证。本文章由计算机程序翻译,如有差异,请以英文原文为准。
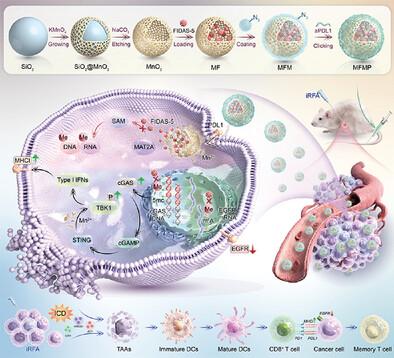
Enhancing Radiofrequency Ablation for Hepatocellular Carcinoma: Nano‐Epidrug Effects on Immune Modulation and Antigenicity Restoration
Radiofrequency ablation (RFA), a critical therapy for hepatocellular carcinoma (HCC), carries a significant risk of recurrence and metastasis, particularly owing to mechanisms involving immune evasion and antigen downregulation via epigenetic modifications. This study introduces a “nano‐epidrug” named MFMP. MFMP, which is composed of hollow mesoporous manganese dioxide (MnO2 ) nanoparticles, FIDAS‐5 as an MAT2A inhibitor, macrophage membrane, and anti‐PD‐L1 (aPD‐L1), targets HCC cells. By selectively binding to these cells, MFMP initially reverses immune suppression via PD‐L1 inhibition. After endocytosis, MFMP disassembles in the tumor microenvironment, releasing FIDAS‐5 and Mn2+ . FIDAS‐5 prevents cGAS methylation, whereas Mn2+ aids STING pathway restoration. In addition, FIDAS‐5 reduces m6 A RNA modification, suppressing EGFR expression. These changes enhance HCC antigenicity to promote cytotoxic T cell recognition and cytotoxic killing. Furthermore, MFMP mediates immunogenic cell death in HCC by synergizing with RFA through cGAS DNA demethylation, EGFR mRNA demethylation, and TBK1 protein phosphorylation, thereby inhibiting recurrence and metastasis and enhancing immune memory. Thus, MFMP is a potential adjunctive therapy requiring clinical validation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Advanced Materials
工程技术-材料科学:综合
CiteScore
43.00
自引率
4.10%
发文量
2182
审稿时长
2 months
期刊介绍:
Advanced Materials, one of the world's most prestigious journals and the foundation of the Advanced portfolio, is the home of choice for best-in-class materials science for more than 30 years. Following this fast-growing and interdisciplinary field, we are considering and publishing the most important discoveries on any and all materials from materials scientists, chemists, physicists, engineers as well as health and life scientists and bringing you the latest results and trends in modern materials-related research every week.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


