通过在 Co0.2FeOx 上构建表面氧空位改善过硫酸盐活化,实现持久的水净化
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
活化过一硫酸盐(PMS)生成硫酸根(SO4--)是一种有效的水净化方法。高效、低成本、易合成的催化剂可确保净化性能和实际应用的可行性。本研究通过在 α-FeOOH 中用 Co2+ 同构取代 Fe3+ 合成了富氧空位磁性 Co0.2FeOx,并将其用于激活 PMS 以降解碘己醇(一种在水中广泛检测到的碘化 X 射线造影剂)。与之前报道的过渡金属氧化物相比,Co0.2FeOx 表现出更高的性能。与单独使用 PMS 氧化相比,在 Co0.2FeOx 催化的体系中,从 pH 值 3.5 到 10.5 的碘己醇都能得到有效降解。利用 ATR-FTIR 光谱在 D2O 和 H2O 中进行的原位测试推断,Co0.2FeOx 表面的氧空位可促进表面羟基的形成,从而与 HSO5- 复合形成 Me-(OH)-OSO3-。通过 Co(II)/Co(III) 和 Fe(III)/Fe(II) 的氧化还原,内部复合物中的电子转移导致 O-O 键断裂,从而促进了自由基的生成。ESR 光谱发现 SO4-- 和 -OH 是主要的活性物种。这项研究为合成高效催化剂提供了新思路,并阐明了 PMS 活化界面机理的新见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
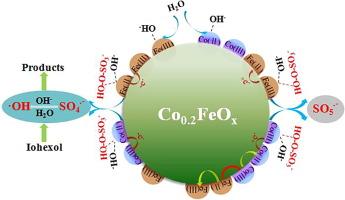
Improving peroxymonosulfate activation via surface-constructed oxygen vacancies on Co0.2FeOx for durable water decontamination
Activating peroxymonosulfate (PMS) to generate sulfate radicals (SO4·-) is an effective method for water purification. An efficient, low-cost, and easily synthesized catalyst ensures decontamination performance and practical application feasibility. In this work, oxygen vacancy-enriched magnetic Co0.2FeOx was synthesized by isomorphous substitution of Fe3+ with Co2+ in α-FeOOH and employed to activate PMS to degrade iohexol, an iodinated X-ray contrast media widely detected in water. Co0.2FeOx exhibited higher performance than previously reported transition metal oxides. Iohexol could be effectively degraded in the Co0.2FeOx-catalyzed system from pH 3.5 to 10.5 compared to PMS oxidation alone. In situ tests in D2O and H2O using ATR-FTIR spectra inferred that the oxygen vacancies on the Co0.2FeOx surface could facilitate the formation of surface hydroxyl groups, which could complex HSO5- to form Me-(OH)-OSO3-. Electron transfer in the inner complex via the redox of Co(II)/Co(III) and Fe(III)/Fe(II) caused the breakage of the O-O bonds, thus promoting free radical generation. ESR spectra identified SO4·- and ·OH as the predominant active species. This study suggests new ideas for the synthesis of efficient catalysts and elucidates new insights into the interfacial mechanism of PMS activation
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


