利用核磁共振和红外分析对米诺地尔进行伏安检测和综合电化学研究:在法医和制药领域的应用。
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
米诺地尔(MN)是一种血管扩张剂,用于治疗脱发和严重高血压。然而,在多个国家都有将其非法用于化妆品和药物制剂的报道。一种高效的米诺地尔检测方法对法医和制药应用具有重大意义。据报道,电化学传感器是检测各种样品中 MN 的一种有趣的替代方法。然而,我们仍需要对氧化还原过程进行更深入的研究,并对 MN 进行更具选择性的电化学检测。在此背景下,我们首次利用核磁共振和傅立叶变换红外光谱分析来了解 MN 在掺硼金刚石电极 (BDDE) 上进行电解后的电化学行为。利用这些综合技术,我们提出并证实了 MN 在掺硼金刚石电极上所有氧化还原过程的电化学机制,其中在磷酸盐缓冲液(pH 值为 6.0)中,出现了 +0.72 V 和 +0.97 V 对(Ag/AgCl/饱和氯化钾)的两个氧化过程。MN 氧化作用产生的最后一种产物在 BDDE 表面以准可逆的氧化还原过程还原为 -0.01 V。利用这一氧化还原过程是在 BDDE 上对 MN 进行选择性和灵敏检测的策略。这种创新方法被成功应用于检测掺假化妆品和药物制剂中的 MN,结果表明,使用相同的 BDDE,检测限低(5.7 µmol.L-1),电化学反应稳定性高(RSD < 1.5 %,n = 6)。因此,所提出的方法为药物和法医样品中 MN 的鉴定和定量提供了一种简单、快速和选择性强的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
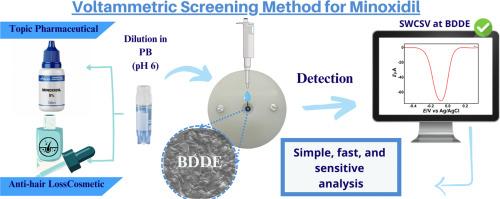
Voltammetric detection with a comprehensive electrochemistry study of minoxidil using nuclear magnetic resonance and infrared analyses: Applications in the forensic and pharmaceutical fields.
Minoxidil (MN) is a vasodilator used to treat hair loss and severe hypertension. However, its illegal use in cosmetics and pharmaceutical formulations has been reported in several countries. An efficient method for MN detection is of great interest for forensic and pharmaceutical applications. Electrochemical sensors have been reported as an interesting alternative for MN detection in various samples. However, a more in-depth study of the redox processes and a more selective electrochemical detection for MN are still required. In this context, we present, for the first time, the use of nuclear magnetic resonance and Fourier transform infrared spectroscopy analyses for understanding the electrochemical behaviour of MN after electrolysis procedures on a boron-doped diamond electrode (BDDE). Using these combined techniques, we have proposed and confirmed an electrochemical mechanism for all redox processes of MN on a BDDE, where in phosphate buffer (pH 6.0) two oxidation processes at +0.72 V and +0.97 V vs (Ag/AgCl/ sat. KCl) are presented. The last generated product by MN oxidation is reduced on the BDDE surface at -0.01 V with a quasi-reversible redox process. The use of this redox process is the strategy for a selective and sensitive detection of MN on the BDDE. This innovative approach was successfully applied to determine MN in adulterated cosmetics and pharmaceutical formulations, showing a low limit of detection (5.7 µmol. L-1) and high stability of electrochemical responses (RSD < 1.5 %, n = 6) using the same BDDE. Therefore, the proposed method provides a simple, fast and selective method for the identification and quantification of MN in pharmaceutical and forensic samples.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


