游离碱和锌(II)三联吡啶卟啉的亲水-亲油平衡以及照射波长对黑色素瘤细胞系光导疗法的影响
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
以往的大量研究表明,卟啉在用作光动力疗法(PDT)的光敏剂(PSs)时,其两亲性和不对称结构是一个非常重要的特性,有利于卟啉进入细胞,从而提高其在 PDT 中的效率。在这项工作中,我们研究了两组阳离子 AB3 吡啶卟啉(游离基和与 Zn(II)螯合)在黑色素瘤细胞系的光动力疗法中的作用,这两组卟啉都被不同长度的烷基链取代。目的是研究亲水-亲油平衡和 Zn(II)螯合的影响,以及使照射波长与 PS 的光学特性相匹配对体外光导疗法效率的重要性。因此,我们对这两个系列的卟啉进行了光谱研究、单线态氧的产生、亲油性以及细胞摄取、定位和细胞毒性研究。在这两个系列的卟啉中,最长的烷基链(17 个 C 原子长)使 PS 的内化程度最高。与 Zn(II)螯合后,卟啉的理化性质更好,但细胞内化速度更慢。正如预期的那样,在低强度(2 mW/cm2)红光(643 nm)下光激活 30 分钟后,游离基卟啉的 PDT 效能高于其 Zn(II) 复合物。不过,在相同通量下使用橙色光(606 纳米)更适合锌(II)卟啉,其总体毒性与亲脂性相似的游离碱类似物类似。虽然带有最长烷基链的 PS 具有最高的光毒性,但 TMPyP3-C13H27 和 Zn(II)-TMPyP3-C13H27 被证明是最有希望用于光导疗法的候选物质,因为它们不仅具有高光毒性,而且与成纤维细胞(HDF)相比,对黑色素瘤细胞系(MeWo 和 A375)具有更大的选择性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
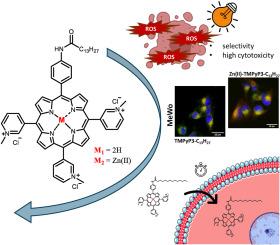
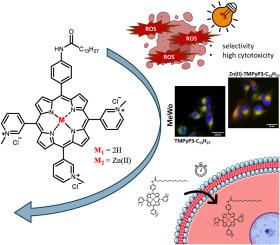
Impact of the hydrophilic-lipophilic balance of free-base and Zn(II) tricationic pyridiniumporphyrins and irradiation wavelength in PDT against the melanoma cell lines
The amphiphilic and asymmetric structure of porphyrins, when used as photosensitizers (PSs) for photodynamic therapy (PDT), has been shown through numerous previous studies to be a very important property that facilitates their entry into cells, which improves their efficiency in PDT. In this work, two groups of cationic AB3 pyridiniumporphyrins, free-base and chelated with Zn(II), both substituted with alkyl chains of various lengths, were studied in PDT on melanoma cell lines. The aim was to investigate the impact of hydrophilic-lipophilic balance and Zn(II) chelation, and the importance of matching the irradiation wavelength to the optical properties of the PS on in vitro PDT efficiency. Therefore, spectroscopic studies, singlet oxygen production and lipophilicity as well as cellular uptake, localization and cytotoxicity studies of the two series of porphyrins were performed. In both series of porphyrins, the longest alkyl chain (17 C-atoms long) enables the greatest internalization of the PS. Chelation with Zn(II) resulted in better physicochemical properties, but slower cellular internalization. As expected, free-base porphyrins were more PDT efficient than their Zn(II) complexes after 30-min photoactivation by low-fluence (2 mW/cm2) red light (643 nm). However the use of orange light (606 nm) with the same fluence rate was more suitable for Zn(II) porphyrins and resulted in similar overall toxicity to their free-base analogues with similar lipophilicity. Although the highest phototoxicity was achieved with the PSs carrying the longest alkyl chain, TMPyP3-C13H27 and Zn(II)-TMPyP3-C13H27 proved to be the most promising candidates for use in PDT as they exhibit high phototoxicity, but also greater selectivity towards melanoma cell lines (MeWo and A375) compared to fibroblasts (HDF).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


