多位质子耦合电子转移氧化分子电化学介质
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
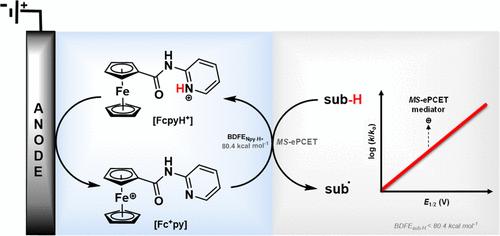
质子耦合电子转移(PCET)为电化学转换提供了一条动力学上有利的途径。受此启发,本研究报道了一种电化学介质--N-吡啶基二茂铁甲酰胺(Fcpy)及其类似物,该介质具有独立的电子和质子转移位点。据估计,Fcpy 介质的 BDFE 为 80.4 kcal mol-1。作为概念验证研究,汉茨酯(HE)的 C-H BDFE 为 70.70 kcal mol-1,经电化学氧化后可得到 93% 的所需产物。计算数据表明,以 Fcpy 为介质的 HE 氧化过程是一个 ET-PCET-PT 过程。此外,还记录了一系列不含任何布氏硬度碱且具有不同 E1/2 的二茂铁衍生物的电化学 HE 氧化动力学,并与 Fcpy 及其类似物进行了比较。电化学 HE 氧化的对数(速率)与 E1/2 的关系表明,多位 PCET 介质具有明显的动力学优势。艾林分析显示了 MS-PCET 介质的关键活化参数。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Molecular Electrochemical Mediator for Oxidative Multi-Site Proton Coupled Electron Transfer
Proton-coupled electron transfer (PCET) allows a kinetically favorable pathway for electrochemical conversions. Inspired by this, an electrochemical mediator, N-pyridylferrocenecarboxamide (Fcpy), having site-separated electron and proton transfer sites and its analog are reported. The BDFE of the Fcpy mediator is estimated to be 80.4 kcal mol–1. As a proof-of-concept study, Hantzsch ester (HE) having a C–H BDFE of 70.70 kcal mol–1 has been electrochemically oxidized to yield 93% of the desired product. The computational data suggests an ET-PCET-PT process for the mediated HE oxidation with Fcpy. Further, the electrochemical HE oxidation kinetics is recorded for a series of ferrocene derivatives devoid of any Brønsted base and having different E1/2 and is compared with the Fcpy and its analog. The logarithm (rate) vs E1/2 for electrochemical HE oxidation shows a clear kinetic advantage for the multisite PCET mediators. Eyring analysis revealed crucial activation parameters for the MS-PCET mediator.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


