烯与磺酰胺的光敏化 1,2 磺化反应
IF 1.9
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在此,我们开发了一种无金属、光敏化的烯烃原料双官能化策略,通过一种量身定制、易于获得的磺酰酰胺双官能试剂来获得 β-氨基砜结构。该方案适用于多种烯烃和多种磺酰酰胺,具有高产率和优异的区域选择性。由于反应条件非常温和、起始材料易于获得、官能团耐受性极佳且具有明确的区域选择性,这种方法将为组装高密度官能化分子支架提供一个高效平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
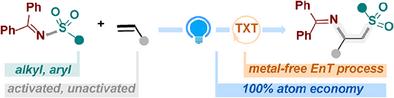
Photosensitized 1,2-Sulfonylamination of Alkenes With Sulfonylamide
Herein, we developed a metal-free, photosensitized strategy for difunctionalization of olefin feedstocks to access β-amino sulphone architectures, enabled by a tailor-made, readily accessible sulfonylamide bifunctional reagent. This protocol could accommodate a wide range of alkenes and a variety of sulfonylamides, with high yields and excellent regioselectivity. Given remarkably mild reaction conditions, readily accessible starting materials, excellent functional group tolerance, and well-defined regioselectivity, this approach would enable an efficient platform for the assembly of densely functionalized molecular scaffolds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ChemistrySelect
Chemistry-General Chemistry
CiteScore
3.30
自引率
4.80%
发文量
1809
审稿时长
1.6 months
期刊介绍:
ChemistrySelect is the latest journal from ChemPubSoc Europe and Wiley-VCH. It offers researchers a quality society-owned journal in which to publish their work in all areas of chemistry. Manuscripts are evaluated by active researchers to ensure they add meaningfully to the scientific literature, and those accepted are processed quickly to ensure rapid online publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


