使用碘代二乙酸苯酯将受体-供体酰肼无金属氧化成重氮化合物
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
The Journal of Organic Chemistry
Pub Date : 2024-10-29
DOI:10.1021/acs.joc.4c0189310.1021/acs.joc.4c01893
引用次数: 0
摘要
芳基酯酰肼很容易在甲醇中与碘代二乙酸苯酯(PIDA)发生反应,生成相应的 α-二氮杂环戊烯酯,而且收率良好甚至极高(30 个实例)。这些条件在合成三唑并吡啶时也被证明是有效的。含有重氮化合物和乙酸的粗混合物也可以用低能量的蓝色 LED 光照射,然后用 AcOH 一锅插入原位生成的碳烯,以高产率得到相应的乙酸盐。本文章由计算机程序翻译,如有差异,请以英文原文为准。
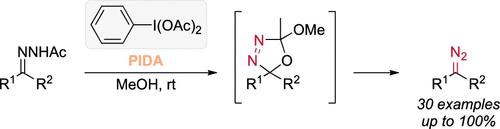
Metal-Free Oxidation of Acceptor–Donor Acylhydrazones into Diazo Compounds Using Phenyl Iododiacetate
Aryl-ester acylhydrazones readily react with phenyl iododiacetate (PIDA) in methanol to produce the corresponding α-diazoesters with good to excellent yields (30 examples). The conditions have also been proven to be efficient in the synthesis of triazolopyridines. The crude mixture containing the diazo compound and acetic acid was also irradiated with low-energy blue LED light for a subsequent one-pot insertion of the in situ-generated carbene with AcOH to afford the respective acetates in high yields.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


