开发用于治疗乳腺癌的口服生物型 PROTAC 雌激素受体降解剂 Vepdegestrant 的商业制造工艺
IF 3.5
3区 化学
Q2 CHEMISTRY, APPLIED
Organic Process Research & Development
Pub Date : 2024-11-05
DOI:10.1021/acs.oprd.4c0036210.1021/acs.oprd.4c00362
引用次数: 0
摘要
为了满足临床和商业需求,我们开发出了一种用于生产 vepdegestrant (1) 的商业工艺,这是一种正在进行人体临床试验的最先进的 PROTAC 蛋白降解剂。该工艺的特点是通过两种先进的手性中间体的最终还原胺化,采用高效的聚合合成策略,以及几种高效的伸缩工艺和稳健的结晶来控制纯度。维替孕甾 (1) 的最终商业化工艺包括七个建议的 GMP 监管步骤和五个分离步骤,总产率为 29%。本文章由计算机程序翻译,如有差异,请以英文原文为准。
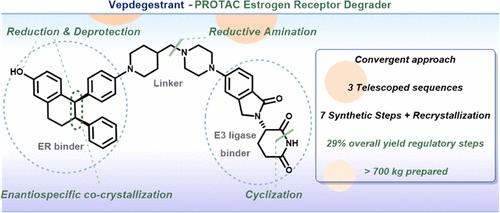
Development of a Commercial Manufacturing Process for Vepdegestrant, an Orally Bioavailable PROTAC Estrogen Receptor Degrader for the Treatment of Breast Cancer
A commercial process for vepdegestrant (1), the most advanced PROTAC protein degrader in human clinical trials, has been developed to support clinical and commercial needs. The process features an efficient convergent synthetic strategy through the final reductive amination of two advanced chiral intermediates, as well as several highly efficient telescoped processes and robust crystallization for purity control. The final commercial process of vepdegestrant (1) consists of seven proposed regulatory GMP steps with five isolations in an overall yield of 29%.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


