gem-Dihalocyclopropanes 的可切换分歧电化学加氢脱卤反应
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
The Journal of Organic Chemistry
Pub Date : 2024-10-29
DOI:10.1021/acs.joc.4c0174810.1021/acs.joc.4c01748
引用次数: 0
摘要
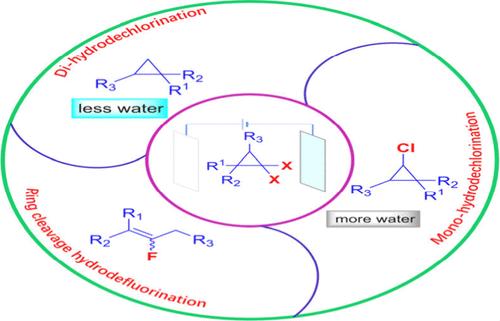
该研究介绍了一种全面有效的电化学方法,可在均匀的电化学条件下对宝石-二氯环丙烷进行多种加氢脱氯反应,并对宝石-二氟环丙烷进行裂环加氢脱氟反应。此外,水含量允许以优异的化学选择性对 gem-dichlorocyclopropanes 进行可调的单氢脱氯或二氢脱氯。此外,该方案的温和性和实用性还有助于将其应用于生物活性分子的后期功能化。机理分析表明,质子源可能来自乙腈。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Switchable Divergent Electrochemical Hydrodehalogenation of gem-Dihalocyclopropanes
A comprehensive and effective electrochemical methodology is introduced for the diverse hydrodechlorination of gem-dichlorocyclopropanes and the ring cleavage hydrodefluorination of gem-difluorocyclopropanes under uniform electrochemical conditions. Moreover, the water content allows for the adjustable monohydrodechlorination or dihydrodechlorination of gem-dichlorocyclopropanes with exceptional chemoselectivity. Additionally, the mildness and practicality of this protocol facilitate its application to the late-stage functionalization of bioactive molecules. Mechanistic analyses suggest that the proton source may originate from acetonitrile.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


