铜催化单锅合成 N,N-4-三苯基噻唑-2-胺
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2024-10-28
DOI:10.1021/acs.joc.4c0141710.1021/acs.joc.4c01417
引用次数: 0
摘要
在此,我们报告了一种以溴苯乙酮、苯基硫脲和碘苯为原料合成 N,N-4-三苯基噻唑-2-胺的高效铜催化策略。该方法具有官能团耐受性好、起始原料易得、操作简单等特点,为 2-氨基噻唑的合成提供了另一种方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
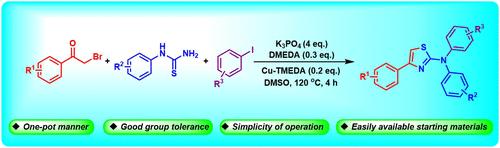
Copper-Catalyzed One-Pot Synthesis of N,N-4-Triphenylthiazol-2-amines
Herein, we reported an efficient copper-catalyzed strategy for the synthesis of N,N-4-triphenylthiazol-2-amines from bromoacetophenone, phenylthiourea and iodobenzene. This method features good functional group tolerance, easy availability of starting materials and simplicity of operation, which provides an alternative method for the synthesis of 2-aminothiazoles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


