用于 DNA 编码文库合成的 DNA 上曼尼希反应
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2024-11-01
DOI:10.1021/acs.joc.4c0209810.1021/acs.joc.4c02098
引用次数: 0
摘要
通过曼尼希反应生成的 β-氨基酮具有作为各种候选药物的巨大潜力。在本研究中,我们优化了 DNA 上曼尼希反应条件,并将其应用于研究 DNA 键合醛与各种胺和酮构建模块的反应。所开发的 DNA 上曼尼希反应保持了 DNA 的完整性,并为文库的生产建立了可行的途径。这些结果凸显了曼尼希反应在 DNA编码文库(DEL)合成中的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
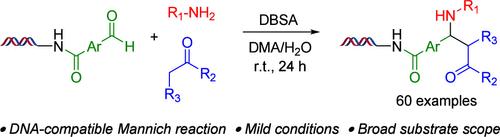
On-DNA Mannich Reaction for DNA-Encoded Library Synthesis
The β-amino ketones produced through the Mannich reaction hold significant potential as candidates for various drugs. In this study, we optimized on-DNA Mannich reaction conditions and applied them to investigate the reactions of DNA-conjugated aldehydes with various amine and ketone building blocks. The developed on-DNA Mannich reaction preserved the DNA integrity and established viable routes for library production. These results underscore the potential of the Mannich reaction in DNA-encoded library (DEL) synthesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


