双(氨基烷基)环亚丙烯基(BAC)催化的 2-(2-甲酰基芳基)芳基取代对醌甲苷与 9-菲醇衍生物的分子内嵌反应
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
The Journal of Organic Chemistry
Pub Date : 2024-10-25
DOI:10.1021/acs.joc.4c0199310.1021/acs.joc.4c01993
引用次数: 0
摘要
本文介绍了在双(氨基烷基)环亚丙烯基(BAC)催化下,通过对战略性设计的 2-(2-甲酰基芳基)苯基取代的对醌甲酰胺(p-QMs)进行分子内环化,从而获得 9-菲醇衍生物和相关碳环。此外,在合成以白藜芦醇为基础的天然产物 (±)-shoreaphenol 和 (±)-malibatol A 的七元碳环核心时,也证明了这种方法的合成效用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
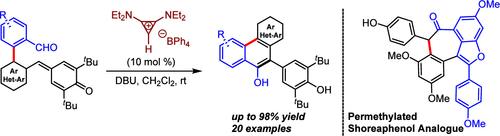
Bis(aminoalkyl)cyclopropenylidene (BAC)-Catalyzed Intramolecular Annulation of 2-(2-Formylaryl)-aryl-Substituted p-Quinone Methides to 9-Phenanthrol Derivatives
This article describes a bis(aminoalkyl)cyclopropenylidene (BAC)-catalyzed intramolecular annulation of strategically designed 2-(2-formylaryl)-phenyl-substituted p-quinone methides (p-QMs) to access 9-phenanthrol derivatives and related carbocycles. In addition, the synthetic utility of this methodology has been demonstrated in the synthesis of the seven-membered carbocyclic core of resveratrol-based natural products (±)-shoreaphenol and (±)-malibatol A.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


