发现调节和立体选择性构建三唑乙烯基磺酰氟的合成枢纽
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
The Journal of Organic Chemistry
Pub Date : 2024-11-01
DOI:10.1021/acs.joc.4c0218610.1021/acs.joc.4c02186
引用次数: 0
摘要
研究人员开发了一种新型磺酰氟试剂 1-溴丁-3-烯-1,3-二磺酰二氟化物(BEDF)。这种独特的试剂具有两种可点击官能团,可用于叠氮-炔环化点击反应和 SuFEx 点击反应。这种新试剂被用于区域选择性地构建一类新型三唑乙烯磺酰氟,其中 C-4 位的 1H-1,2,3-三唑被完全 E 构型的乙烯磺酰氟官能化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
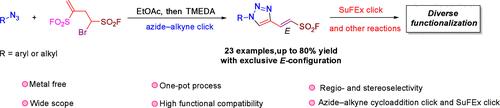
Discovery of A Synthetic Hub for Regio- and Stereoselective Construction of Triazolyl Vinyl Sulfonyl Fluorides
A new sulfonyl fluoride reagent 1-bromobut-3-ene-1,3-disulfonyl difluoride (BEDF) was developed. This unique reagent possesses two clickable functionalities to be used for both azide–alkyne cycloaddition click and SuFEx click reactions. This new reagent was applied for the regioselective construction of a class of novel triazolyl vinyl sulfonyl fluorides in which the C-4 position 1H-1,2,3-triazoles were functionalized with vinyl sulfonyl fluorides of exclusively E-configuration.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


