通过串联迈克尔加成/5-外-二元环化反应非对映选择性地构建螺环状异苯并呋喃
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2024-10-31
DOI:10.1021/acs.joc.4c0189010.1021/acs.joc.4c01890
引用次数: 0
摘要
本文介绍了一种快速有效地获得螺环异苯并呋喃的实用方法。该反应通过 1,6-炔酮衍生物和 4-硝基-1,3-二芳基丁-1-酮的环加成反应进行,在 l-脯氨酸作为催化剂存在的情况下,Cs2CO3 起促进作用。该反应的优点包括形成两个 C-C 键和一个 C-O 键,以及反应条件温和。在这种温和的条件下,可以高效、非对映选择性地获得扩展的螺环异苯并呋喃,而且这种新方案避免了使用任何过渡金属试剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
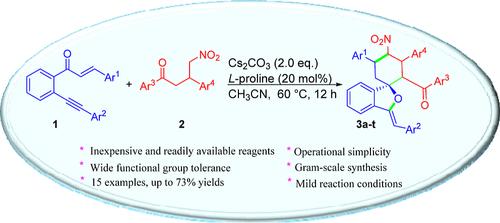
Diastereoselective Construction of Spirocyclic Isobenzofurans via a Tandem Michael Addition/5-Exo-dig Cyclization Reaction
A practical approach for rapid and efficient access to spirocyclic isobenzofurans is described. The reaction proceeds through the cycloaddition of 1,6-ynenone derivatives and 4-nitro-1,3-diarylbutan-1-ones, promoted by Cs2CO3 in the presence of l-proline as a catalyst. The advantages of this reaction include the formation of two C–C bonds and one C–O bond as well as mild reaction conditions. Extended spirocyclic isobenzofurans are obtained with good efficiency and diastereoselectivity under these mild conditions, and this new protocol avoids the use of any transition-metal reagents.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


