连续流镍或钌沸石催化二元醇和仲酸分子内脱水制备大环醚和内酯
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
The Journal of Organic Chemistry
Pub Date : 2024-10-24
DOI:10.1021/acs.joc.4c0169210.1021/acs.joc.4c01692
引用次数: 0
摘要
在此,我们报告了在连续流模块下,以镍沸石为催化剂,对称和不对称二元醇分子内脱水偶联合成大环冠醚的高效方法。这种方法对于使用镍沸石或 Ru 沸石进行仲酸的分子内脱水大内酯化也很有效。这种流动催化方法在广泛的底物范围内通过一次性填料镍沸石生产出 20 种大环聚醚和 11 种以水为副产物的广义大内酯。本文章由计算机程序翻译,如有差异,请以英文原文为准。
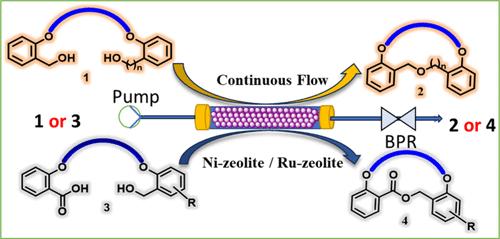
Continuous Flow Ni- or Ru-Zeolite-Catalyzed Intramolecular Dehydration of Diols and Seco-Acids for Macrocyclic Ethers and Lactones
Herein, we report the efficient intramolecular dehydrative coupling of symmetrical and unsymmetrical diols for the synthesis of macrocyclic crown ethers in the presence of Ni-zeolite as a catalyst under continuous flow module. This method is also efficient for the intramolecular dehydrative macrolactonization of seco-acids using Ni-zeolite or Ru-zeolite. This flow catalysis is demonstrated by a wide substrate scope with one-time packed Ni-zeolite to produce 20 macrocyclic polyethers and 11 examples of broad macrolactones with water as a byproduct.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


