室温下铑催化吲哚与芳基硅烷的 C-H 芳基化反应
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
The Journal of Organic Chemistry
Pub Date : 2024-11-06
DOI:10.1021/acs.joc.4c0154910.1021/acs.joc.4c01549
引用次数: 0
摘要
我们开发了 Cp*Rh 催化吲哚与芳基硅烷的 C-H 芳基化反应。这种 C-H 活化转化使 Rh 催化的吲哚 C2 芳基化克服了需要强引导基辅助和高温条件的限制,实现了由弱引导基驱动的室温转化。Cp*Rh/MeOH 催化介质被认为是使这一转化在温和条件下发生的关键因素,实验研究和理论计算旨在合理解释反应机理以及甲醇作为溶剂对促进反应的影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
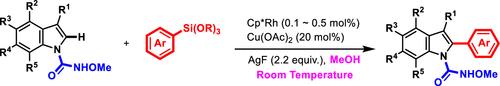
Rhodium-Catalyzed C–H Arylation of Indoles with Arylsilanes at Room Temperature
The Cp*Rh-catalyzed C–H arylation of indoles with arylsilanes is developed. This C–H activation transformation allows for the Rh-catalyzed indole C2 arylation to overcome the limitations of requiring strong directing group assistance and high-temperature conditions, achieving a room-temperature transformation driven by a weak directing group. Cp*Rh/MeOH catalytic media are considered a key factor enabling this transformation to occur under mild conditions, and experimental studies and theoretical calculations were performed to rationalize the reaction mechanisms and the influence of methanol as a solvent in promoting the reaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


