Ru 催化 N-氯苯甲酰胺与不对称炔烃在水中的氧化还原中性偶联反应
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
The Journal of Organic Chemistry
Pub Date : 2024-10-24
DOI:10.1021/acs.joc.4c0223410.1021/acs.joc.4c02234
引用次数: 0
摘要
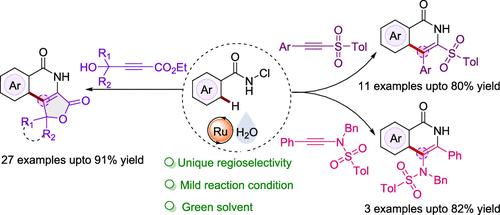
在室温、无氧化剂的外部条件下,N-氯苯甲酰胺与带有芳基、羟基、酯和磺酰官能团的不对称内炔在水中通过 Ru 催化环化反应得到了异喹啉酮支架。使用水作为反应介质、氧化还原中性条件、区域选择性和底物范围是该研究的重要实用特征。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Ru-Catalyzed Redox-Neutral Coupling of N-Chlorobenzamides with Unsymmetrical Alkynes in Water
In water, Ru-catalyzed annulation of N-chlorobenzamides with unsymmetrical internal alkynes bearing aryl, hydroxy, ester, and sulfonyl functionalities has been accomplished to afford isoquinolone scaffolds under external oxidant-free conditions at room temperature. Use of water as reaction medium, redox-neutral conditions, regioselectivity, and substrate scope are important practical features.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


