有机光氧催化乙烯基硼酸酯的氢硅烷化作用以合成双子和双子硼硅烷
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
The Journal of Organic Chemistry
Pub Date : 2024-10-24
DOI:10.1021/acs.joc.4c0173110.1021/acs.joc.4c01731
引用次数: 0
摘要
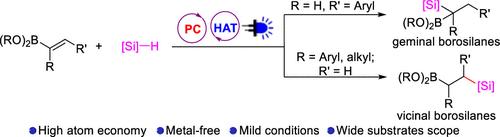
宝石硼硅烷和沧基硼硅烷在功能材料和合成转化中有着独特的应用。在此,我们通过乙烯基硼酸酯的光氧化无金属氢硅烷化反应,开发了一种合成geminal和vicinal硼硅烷的简便方法。该方法具有原子经济性高、环境友好、官能团相容性好等优点。机理研究表明,催化反应经历了光氧化 HAT 催化和自由基加成途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Organic Photoredox-Catalyzed Hydrosilylation of Vinylboronic Esters for the Synthesis of Geminal and Vicinal Borosilanes
Geminal and vicinal borosilanes have unique applications in functional materials and synthetic transformations. Herein, a convenient method for the synthesis of geminal and vicinal borosilanes is developed via photoredox metal-free hydrosilylation of vinylboronic esters. This strategy features the advantages of high atom economy, environmental friendliness, and excellent functional group compatibility. The mechanism studies reveal that the catalytic reaction goes through photoredox HAT catalysis and a radical addition pathway.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


