发现微管相关丝氨酸/苏氨酸激酶样 (MASTL) 的高选择性抑制剂
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
Journal of Medicinal Chemistry
Pub Date : 2024-11-05
DOI:10.1021/acs.jmedchem.4c0165910.1021/acs.jmedchem.4c01659
引用次数: 0
摘要
由于微管相关丝氨酸/苏氨酸激酶样(MASTL)在细胞增殖中的作用,它是一个新靶点,也是为肿瘤患者提供有影响力的新治疗药物的一流(FIC)机会。在本文中,我们介绍了一项 "命中到先导 "的优化工作,该工作的成果是提供了两种高选择性的 MASTL 抑制剂。实现这项工作的关键策略包括基于结构的药物设计(SBDD)、亲脂效率分析(LipE)和新型合成。由此产生的先进先导化合物促成了一项肿瘤生长抑制研究,该研究对于评估 MASTL 作为肿瘤治疗靶点的潜在价值至关重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
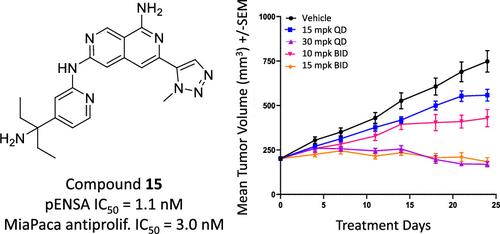
Discovery of Highly Selective Inhibitors of Microtubule-Associated Serine/Threonine Kinase-like (MASTL)
By virtue of its role in cellular proliferation, microtubule-associated serine/threonine kinase-like (MASTL) represents a novel target and a first-in-class (FIC) opportunity to provide a new impactful therapeutic agent to oncology patients. Herein, we describe a hit-to-lead optimization effort that resulted in the delivery of two highly selective MASTL inhibitors. Key strategies leveraged to enable this work included structure-based drug design (SBDD), analysis of lipophilic efficiency (LipE) and novel synthesis. The resulting advanced lead compounds enabled a tumor growth inhibition study which was pivotal in assessing the potential value of MASTL as an oncology therapeutic target.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


