铁介导的烯丙基醚与苄基溴的二烷基化反应
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
The Journal of Organic Chemistry
Pub Date : 2024-04-04
DOI:10.1021/acs.joc.3c0254810.1021/acs.joc.3c02548
引用次数: 0
摘要
我们公开了一种利用铁粉使烯基烯丙基醚与溴化苄基醚发生二苄基化反应的方法。该反应通过在烯烃的邻位碳上形成两个新的 C(sp3)-C(sp3) 键,生成缀有功能化芳基环的支链烷基支架。该方案可容忍富电子、中性电子和贫电子苄基溴和烯丙基烯。机理研究表明,苄基自由基中间体的形成是铁的单电子转移的结果,这种转移被烯基烯烃截获。本文章由计算机程序翻译,如有差异,请以英文原文为准。
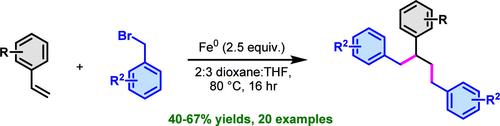
Iron-Mediated Dialkylation of Alkenylarenes with Benzyl Bromides
We disclose a method for the dibenzylation of alkenylarenes with benzyl bromides using iron powder. This reaction generates branched alkyl scaffolds adorned with functionalized aryl rings through the formation of two new C(sp3)–C(sp3) bonds at the vicinal carbons of alkenes. This protocol tolerates electron-rich, electron-neutral, and electron-poor benzyl bromides and alkenylarenes. Mechanistic studies suggest the formation of benzylic radical intermediates as a result of single-electron transfer from the iron, which is intercepted by alkenylarenes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


