在城市固体废物焚化炉底灰的支持下,通过热化学转化实现聚碳酸酯废料的原料回收。
IF 8.1
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
不断增长的塑料需求推动了塑料生产,造成了二氧化碳排放和微塑料污染等严峻的环境挑战。此外,城市固体废物(MSW)处理过程中产生的副产品--焚化炉底灰(IBA)的不当处置也带来了额外的环境风险。本研究探索了一种从塑料产品中回收非石油基单体的方法。本研究使用智能手机外壳废料(SCW)作为原料,经热重分析和傅里叶变换红外光谱分析证实,该废料由聚碳酸酯(PC)制成。城市固体废物焚烧炉底灰(MSW-IBA)被用作 SCW 热化学转化的催化剂。为了确定回收双酚 A 的最佳热解条件,实验在不同的气氛(N₂ 和 CO₂)和催化剂配置(原位和非原位)下进行。与非催化转化相比,MSW-IBA 在 600 °C 的氮气环境下可将 PC 的单体双酚 A(BPA)的产量提高 127%。与原位配置相比,原位配置催化剂负载可使双酚 A 产量提高 147%。SCW 的双酚 A 产量增加很可能是因为 MSW-IBA 催化剂上的金属氧化物(如 CaO)促进了 C-O 键的裂解、CO(或 CO2)的解离以及从 C1-C3 碳氢化合物和 H2 中提取氢气。在二氧化碳环境下催化转化 SCW 时,二氧化碳会吸附到 MSW-IBA 中的 CaO 上,从而减少活性位点的数量。它使催化剂失活,导致双酚 A 收率(22.96 wt%)低于在 N2 气氛下获得的双酚 A 收率(25.86 wt%)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
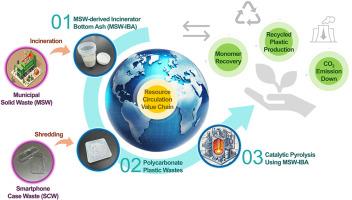
Feedstock recycling of polycarbonate waste via thermochemical conversion supported by municipal solid waste incinerator bottom ash
The rising demand for plastics has driven up its production, causing severe environmental challenges like CO2 emissions and microplastic pollution. Furthermore, improper disposal of incinerator bottom ash (IBA), a byproduct of municipal solid waste (MSW) treatment, poses additional environmental risks. This study explores a method for recovering non-petroleum-based monomers from plastic products. A smartphone case waste (SCW) is used as feedstock in this study and it is made of polycarbonate (PC), confirmed by thermogravimetric analysis and Fourier transform infrared spectroscopy. The MSW incinerator bottom ash (MSW-IBA) is used as a catalyst for thermochemical conversion of SCW. To determine the optimal pyrolysis conditions for BPA recovery, experiments were conducted under different atmospheres (N₂ and CO₂) and catalyst configurations (in situ and ex situ). The MSW-IBA leads to 127% higher yield of bisphenol A (BPA), the monomer of PC, at 600 °C under a N2 atmosphere, compared to non-catalytic conversion. In situ configuration of the catalyst loading leads to up to 147% higher BPA yield than ex situ configuration. The increased BPA production from SCW is most likely because metal oxides (e.g., CaO) present on the MSW-IBA catalyst promote the cleavage of and C–O bonds, dissociation of CO (or CO2) and hydrogen extraction from C1–C3 hydrocarbons and H2. For the catalytic conversion of SCW under a CO2 atmosphere, CO2 adsorbs onto CaO in the MSW-IBA, decreasing the number of active sites. It deactivates the catalyst, resulting in a lower BPA yield (22.96 wt%) than the BPA yield obtained under the N2 atmosphere (25.86 wt%).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemosphere
环境科学-环境科学
CiteScore
15.80
自引率
8.00%
发文量
4975
审稿时长
3.4 months
期刊介绍:
Chemosphere, being an international multidisciplinary journal, is dedicated to publishing original communications and review articles on chemicals in the environment. The scope covers a wide range of topics, including the identification, quantification, behavior, fate, toxicology, treatment, and remediation of chemicals in the bio-, hydro-, litho-, and atmosphere, ensuring the broad dissemination of research in this field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


