苯丙酮途径在砷胁迫水稻基因型的遗传调控和生理适应中的作用
IF 5.7
2区 生物学
Q1 PLANT SCIENCES
引用次数: 0
摘要
本研究调查了砷污染水稻基因型在自然条件下的苯丙酮途径的作用,探索了遗传调控与生理适应之间错综复杂的关系。通过分析酶活性和相关基因表达模式、对接模拟和营养动态,阐明了水稻基因型为抵御砷暴露而采取的不同方法。苯丙氨酸途径的酶分析表明,不同水稻基因型之间存在显著差异,与 Sampoorna 和 Pioneer 相比,Mini mansoori 的关键酶(PAL、C4H、4CL、CHI、DFR 和 F3H)的活性水平明显更高。此外,基因表达谱分析揭示了不同的反应,与 Sampoorna 相比,Mini mansoori 和 Pioneer 表现出更高的抗砷和耐砷基因(OsPAL、OsCHS、OsCHI、OsF3H、OsF3'H、OsFLS、OsDFR 和 OsLAR)表达量。富集分析强调了肉桂酸生物合成及相关途径的参与。分子对接表明,某些蛋白质(如 Os4CL、OsFLS、OsDFR 和 OsLAR)容易与 As 结合,从而可能影响酶的活性。营养学分析表明,迷你芒梭利能保持较高水平的谷物必需营养素,如 Na、Ca、P、Mn、Mg 和 Zn。然而,这与 "先锋 "和 "桑普尔纳 "形成了鲜明对比,"先锋 "和 "桑普尔纳 "出现了养分失衡,可能是由于砷积累较多。叶绿素荧光分析表明,与 Sampoorna 相比,Mini mansoori 和 Pioneer 在砷中毒的情况下保持了更好的光合效率。此外,网络分析强调了镁和钠与必需酚类物质和类黄酮的相互作用在对抗胁迫中的关键作用。利用这一认识,有针对性的育种工作可培育出营养成分和类黄酮含量更高的抗砷水稻品种,从而解决受影响地区的食品安全和营养不良问题。本文章由计算机程序翻译,如有差异,请以英文原文为准。
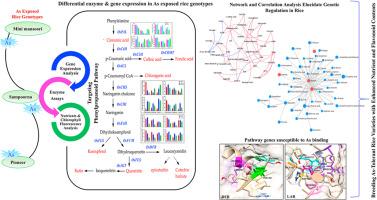
Role of phenylpropanoid pathway in genetic regulation and physiological adaptation in arsenic stressed rice genotypes
This study investigates the role of the phenylpropanoid pathway in arsenic (As) contaminated rice genotypes under natural conditions, exploring the intricate relationship between genetic regulation and physiological adaptation. Differential approaches adapted by rice genotypes to counteract As exposure are elucidated here through analysis of enzyme activities and related gene expression patterns, docking simulations, and nutrient dynamics. Enzymatic analysis from the phenylpropanoid pathway revealed significant variations across rice genotypes, with Mini mansoori exhibiting notably higher activity levels of key enzymes (PAL, C4H, 4CL, CHI, DFR and F3H) compared to Sampoorna and Pioneer. Additionally, the gene expression profiling unveiled differential responses, with Mini mansoori and Pioneer demonstrating higher expression of genes (OsPAL, OsCHS, OsCHI, OsF3H, OsF3′H, OsFLS, OsDFR, and OsLAR) associated with As resistance and tolerance, compared to Sampoorna. Enrichment analysis emphasized the involvement of cinnamic acid biosynthesis and related pathways. Molecular docking depicted certain proteins, such as Os4CL, OsFLS, OsDFR, and OsLAR susceptible to As binding, potentially affecting enzymatic activity. Ionomic analysis unveiled that Mini mansoori maintained higher levels of essential nutrients such as Na, Ca, P, Mn, Mg, and Zn in grains. However, this contrasted with Pioneer and Sampoorna, which experienced nutrient imbalance likely due to higher As accumulation. Chlorophyll fluorescence analysis depicted that Mini mansoori and Pioneer maintained better photosynthetic efficiency under As toxicity compared to Sampoorna. Moreover, network analysis highlights the critical role of Mg and Na interaction with essential phenolics and flavonoids, in combating the stress. Harnessing this understanding, targeted breeding effort could yield As-resistant rice varieties with enhanced nutrient and flavonoid contents, addressing both food safety and malnutrition in affected regions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.10
自引率
3.10%
发文量
410
审稿时长
33 days
期刊介绍:
Plant Physiology and Biochemistry publishes original theoretical, experimental and technical contributions in the various fields of plant physiology (biochemistry, physiology, structure, genetics, plant-microbe interactions, etc.) at diverse levels of integration (molecular, subcellular, cellular, organ, whole plant, environmental). Opinions expressed in the journal are the sole responsibility of the authors and publication does not imply the editors'' agreement.
Manuscripts describing molecular-genetic and/or gene expression data that are not integrated with biochemical analysis and/or actual measurements of plant physiological processes are not suitable for PPB. Also "Omics" studies (transcriptomics, proteomics, metabolomics, etc.) reporting descriptive analysis without an element of functional validation assays, will not be considered. Similarly, applied agronomic or phytochemical studies that generate no new, fundamental insights in plant physiological and/or biochemical processes are not suitable for publication in PPB.
Plant Physiology and Biochemistry publishes several types of articles: Reviews, Papers and Short Papers. Articles for Reviews are either invited by the editor or proposed by the authors for the editor''s prior agreement. Reviews should not exceed 40 typewritten pages and Short Papers no more than approximately 8 typewritten pages. The fundamental character of Plant Physiology and Biochemistry remains that of a journal for original results.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


