甘草酸二铵通过调节肠肝轴减轻铁超载引起的小鼠肝损伤
IF 6.7
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
背景:有证据表明,铁超载(IO)与慢性肝病的发病机制密切相关,这凸显了针对IO的干预措施有可能阻碍或减缓慢性肝病的进展。甘草酸二铵(DG)是甘草的主要成分甘草酸的药用形式,已被临床用作一种保肝剂;然而,它对IO诱导的肝损伤的保护作用及其潜在的分子机制仍未确定。研究目的:本研究旨在探讨甘草酸二铵对IO诱导的肝损伤的保肝作用,重点关注肠肝轴:已在体内建立了 IO 诱导的肝损伤和 DG 治疗的动物模型。对使用右旋糖酐铁或 DG 治疗的小鼠的铁沉积、肝损伤、肠道屏障损伤和肝脏炎症进行了评估。使用 16S rRNA 全长测序分析了粪便中微生物组的组成。采用 UPLC-Q-TOF-MS 技术检测了粪便中胆汁酸(BAs)的含量,并测定了参与胆汁酸代谢的受体、酶或转运体的表达水平:结果:DG能部分减少IO小鼠肝脏中的铁沉积和亚铁离子水平,从而减轻氧化损伤。DG还能改善肠道微生物群失调,修复肠道屏障损伤,抑制内毒素向肝脏的转运,进而抑制TLR4/NF-κB/NLRP3通路介导的IO引起的肝脏炎症。此外,DG 还能调节 IO 小鼠的 BAs 代谢紊乱,减少 BAs 在肝脏中的积累:结论:DG通过调节肠肝轴减轻了IO诱导的小鼠肝损伤。本研究为 DG 改善 IO 引起的肝损伤的内在机制提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
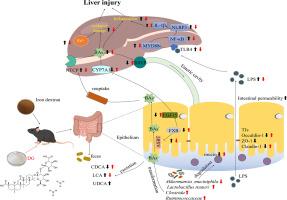
Diammonium glycyrrhizinate alleviates iron overload-induced liver injury in mice via regulating the gut-liver axis
Background
Evidence indicates a close association between iron overload (IO) and the pathogenesis of chronic liver diseases, highlighting the potential for interventions targeted at IO to impede or decelerate the progression of chronic liver diseases. Diammonium glycyrrhizinate (DG), the medicinal form of glycyrrhizic acid, a principal constituent of licorice, has been clinically employed as a hepatoprotective agent; however, its protective effect against IO-induced liver injury and underlying molecular mechanisms remain elusive.
Purpose
The aim of the present study is to investigate the hepatoprotective effect of DG against IO-induced liver injury with a focus on the gut-liver axis.
Study design and methods
Animal models of IO-induced liver injury and DG treatment have been established in vivo. Iron deposition, liver injury, intestinal barrier damage, and liver inflammation were assessed in mice treated with iron dextran or DG. The microbiome composition in feces was analyzed using 16S rRNA full-length sequencing. Bile acids (BAs) profiles in feces were detected by UPLC-Q-TOF-MS technique, and the expression levels of receptors, enzymes or transporters involved in BAs metabolism were also determined.
Results
DG partially reduced the iron deposition and the levels of ferrous ion in the livers of mice with IO, thereby mitigating oxidative damage. DG also improved gut microbiota dysbiosis, repaired intestinal barrier damage, inhibited endotoxin translocation to the liver, and subsequently suppressed TLR4/NF-κB/NLRP3 pathway-mediated liver inflammation caused by IO. Moreover, DG modulated BAs metabolism disorder in IO mice, reducing the accumulation of BAs in the liver.
Conclusion
DG alleviates IO-induced liver injury in mice by regulating the gut-liver axis. This study provides novel insights into the underlying mechanisms through which DG ameliorates liver injury caused by IO.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


