具有 PCSK9 分泌抑制活性的 Combretum quadrangulare Kurz 中的环安坦类三萜。
IF 3.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
采用物理化学和光谱学方法,从 Combretum quadrangulare Kurz 中分离并鉴定了九种以前未曾描述过的(1-9)和七种已知的(10-16)环安坦类三萜类化合物。这些化合物的绝对构型是通过改进的莫舍尔法以及电子圆二色性(ECD)和振动圆二色性(VCD)光谱的量子化学计算确定的。评估了这些化合物对 PCSK9 分泌的抑制活性,并确定了合理的结构-活性关系。化合物 2、14 和 15 对 PCSK9 mRNA 和蛋白质水平具有显著的抑制作用,与阿托伐他汀联合处理时,可观察到明显的 PCSK9 mRNA 抑制作用。化合物 15 的活性最强,与阴性对照组相比,它能显著提高低密度脂蛋白的吸收。体内药代动力学研究证实,化合物 15 在肝脏中的分布高于血浆,因为 PCSK9 主要在肝脏中合成。这些发现强调了环安坦类三萜支架在发现 PCSK9 抑制剂方面的潜在意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
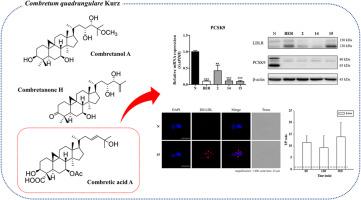
Cycloartane-type triterpenoids from Combretum quadrangulare Kurz with PCSK9 secretion inhibitory activities
Nine previously undescribed (1–9) and seven known (10–16) cycloartane-type triterpenoids were isolated and characterized from Combretum quadrangulare Kurz using physicochemical and spectroscopic methods. The absolute configurations of these compounds were determined through modified Mosher's method and quantum chemical calculation of electronic circular dichroism (ECD) and vibrational circular dichroism (VCD) spectra. Their inhibitory activities against PCSK9 secretion were assessed, and a plausible structure-activity relationship was delineated. Compounds 2, 14, and 15 exhibited notable inhibitory effects on PCSK9 mRNA and protein levels, and significant PCSK9 mRNA inhibition was observed when co-treated with atorvastatin. Compound 15 showed the most potent activity, markedly enhancing LDL uptake compared to the negative control. In vivo pharmacokinetic studies confirmed that compound 15 exhibited higher distribution in the liver than plasma, where PCSK9 is predominantly synthesized. These findings emphasize the potential significance of the cycloartane-type triterpenoid scaffold in discovering PCSK9 inhibitors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytochemistry
生物-植物科学
CiteScore
6.40
自引率
7.90%
发文量
443
审稿时长
39 days
期刊介绍:
Phytochemistry is a leading international journal publishing studies of plant chemistry, biochemistry, molecular biology and genetics, structure and bioactivities of phytochemicals, including ''-omics'' and bioinformatics/computational biology approaches. Phytochemistry is a primary source for papers dealing with phytochemicals, especially reports concerning their biosynthesis, regulation, and biological properties both in planta and as bioactive principles. Articles are published online as soon as possible as Articles-in-Press and in 12 volumes per year. Occasional topic-focussed special issues are published composed of papers from invited authors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


