转染来自扑粉虱 Laodelphax striatellus 的 Wolbachia 对烟粉虱繁殖能力和转录组的影响
IF 3.6
3区 生物学
Q1 ZOOLOGY
引用次数: 0
摘要
烟粉虱是威胁农作物并导致农业损失的重要全球性害虫。沃尔巴克氏菌是昆虫细胞内的一种共生细菌,可以通过多种方式调节宿主的生长和发育。此前的一项研究发现,沃尔巴克氏菌可转移到粉虱体内并诱导其体能变化。然而,人们对烟粉虱寄主与狼杆菌相互作用的内在机制知之甚少。在本研究中,从小褐飞虱 Laodelphex striatellus 中分离出了 Wolbachia 菌株 wStri,并将其转移给了烟粉虱。利用荧光原位杂交技术测定了粉虱体内沃尔巴克氏体的分布情况。互交实验表明,wStri 不会诱导烟粉虱出现细胞质不相容表型,但会延长后代的发育时间。我们利用基于Illumina的RNA-Seq技术对感染了Wolbachia的雌性和雄性成虫进行了转录组分析。在受感染的雌性成虫中,共发现了843个差异表达基因(DEGs),其中141个基因在狼毒感染后显著上调,702个基因在感染后显著下调。在受感染的雄性个体中,511个基因组中有279个宿主基因在感染沃尔巴克氏体后明显上调,232个基因在感染沃尔巴克氏体后明显下调。对 DEGs 的 KEG 分析表明,上调基因和下调基因在基因通路分布上存在明显差异。这些基因参与了各种生物过程,包括但不限于解毒、氧化还原、代谢过程和免疫。这项研究的转录组分析为我们提供了关于沃尔巴克氏体感染后粉虱体内基因差异表达的宝贵信息,并加深了我们对这种宿主-共生相互作用的理解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
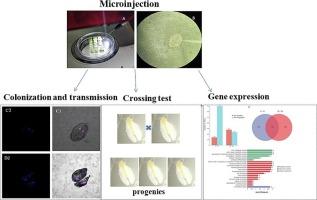
Impact of transinfection of Wolbachia from the planthopper Laodelphax striatellus on reproductive fitness and transcriptome of the whitefly Bemisia tabaci
The whitefly Bemisia tabaci is critical global pest threatening crops and leading to agricultural losses. Wolbachia is an intracellular symbiotic bacterium in insects, which can regulate the growth and development of the host through various ways. In a prior study, Wolbachia was found to be transferred to whitefly and induce fitness changes. However, little is known about the underlying mechanisms of host-Wolbachia interactions in B. tabaci. In this study, a Wolbachia strain wStri was isolated from the small brown planthopper, Laodelphex striatellus, and transferred to B. tabaci. The distribution of Wolbachia in whiteflies was determined using fluorescence in situ hybridization. Reciprocal crossing experiments demonstrated that wStri did not induce cytoplasmic incompatibility phenotypes in B. tabaci, but prolonged the developmental duration of the offspring. We performed transcriptomic analysis of Wolbachia-infected female and male adults using Illumina-based RNA-Seq. A total of 843 differentially expressed genes (DEGs) were identified in infected females, among them 141 were significantly up-regulated and 702 were down-regulated by Wolbachia infection. In infected males, of 511 gene sets, 279 host genes were significantly up-regulated, and 232 were down-regulated by Wolbachia infection. KEGG analysis of DEGs demonstrated significant differences in gene pathway distribution between up-regulated and down-regulated genes. These genes are involved in various biological processes, including, but not limited to, detoxification, oxidation–reduction, metabolic processes, and immunity. The transcriptomic profiling of this study offers valuable information on the differential expression of genes in whiteflies following Wolbachia infection, and enhances our understanding of this host-symbiotic interaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.10
自引率
5.90%
发文量
94
审稿时长
1 months
期刊介绍:
The Journal of Invertebrate Pathology presents original research articles and notes on the induction and pathogenesis of diseases of invertebrates, including the suppression of diseases in beneficial species, and the use of diseases in controlling undesirable species. In addition, the journal publishes the results of physiological, morphological, genetic, immunological and ecological studies as related to the etiologic agents of diseases of invertebrates.
The Journal of Invertebrate Pathology is the adopted journal of the Society for Invertebrate Pathology, and is available to SIP members at a special reduced price.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


