一种三维打印微型装置封装了由源自iPSC的β样细胞和微血管片段组成的血管化小胰岛,用于治疗1型糖尿病。
IF 12.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
胰岛素分泌细胞的移植为重建1型糖尿病(T1D)患者的自主血糖控制能力提供了一种可行的方法,但移植细胞的低存活率阻碍了治疗效果。在这项研究中,我们用三维打印技术制造了一种含有β样细胞和微血管片段(MVF)的封装系统,从而在体内制造出一种带有血管化小胰岛的可重复使用的微型装置,用于治疗T1D。功能性β样细胞由尿液上皮细胞衍生的诱导多能干细胞(UiPSCs)分化而来。单细胞 RNA 测序对整个分化过程进行了综合研究和宏观发育分析,发现体外分化的发育轨迹与体内胚胎胰腺的发育模式一致。MVF是从附睾脂肪垫中分离出来的。利用数字光处理(DLP)-三维打印技术快速制备了具有凹槽结构的微器件。β样细胞和MVF均匀地分布在装置中。将微装置移植到 C57BL/6 小鼠皮下后,胶原堆积较少,免疫细胞浸润较低。此外,在不使用免疫抑制剂的情况下,封装了血管化胰岛的微装置在长达 100 天的治疗中减少了 33% 的小鼠的高血糖症状,而且在小鼠血清中也检测到了人源化 C 肽。总之,我们描述的微装置保护血管化小胰岛用于T1D的长期治疗,具有高度的安全性和潜在的临床转化价值,因此可能为推动T1D的β细胞替代疗法的研究进展提供一种可转化的解决方案。本文章由计算机程序翻译,如有差异,请以英文原文为准。
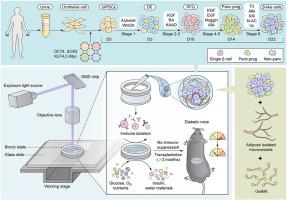
A 3D-printed microdevice encapsulates vascularized islets composed of iPSC-derived β-like cells and microvascular fragments for type 1 diabetes treatment
Transplantation of insulin-secreting cells provides a promising method for re-establishing the autonomous blood glucose control ability of type 1 diabetes (T1D) patients, but the low survival of the transplanted cells hinder the therapeutic efficacy. In this study, we 3D-printed an encapsulation system containing β-like cells and microvascular fragments (MVF), to create a retrivable microdevice with vascularized islets in vivo for T1D therapy. The functional β-like cells were differentiated from the urine epithelial cell-derived induced pluripotent stem cells (UiPSCs). Single-cell RNA sequencing provided an integrative study and macroscopic developmental analyses of the entire process of differentiation, which revealed the developmental trajectory of differentiation in vitro follows the developmental pattern of embryonic pancreas in vivo. The MVF were isolated from the epididymal fat pad. The microdevice with a groove structure were rapidly fabricated by the digital light processing (DLP)-3D printing technology. The β-like cells and MVF were uniformly distributed in the device. After subcutaneous transplantation into C57BL/6 mice, the microdevice have less collagen accumulation and low immune cell infiltration. Moreover, the microdevice encapsulated vascularized islets reduced hyperglycemia in 33 % of the treated mice for up to 100 days without immunosuppressants, and the humanized C-peptide was also detected in the serum of the mice. In summary, we described the microdevice-protected vascularized islets for long-term treatment of T1D, with high safety and potential clinical transformative value, and may therefore provide a translatable solution to advance the research progress of β cell replacement therapy for T1D.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biomaterials
工程技术-材料科学:生物材料
CiteScore
26.00
自引率
2.90%
发文量
565
审稿时长
46 days
期刊介绍:
Biomaterials is an international journal covering the science and clinical application of biomaterials. A biomaterial is now defined as a substance that has been engineered to take a form which, alone or as part of a complex system, is used to direct, by control of interactions with components of living systems, the course of any therapeutic or diagnostic procedure. It is the aim of the journal to provide a peer-reviewed forum for the publication of original papers and authoritative review and opinion papers dealing with the most important issues facing the use of biomaterials in clinical practice. The scope of the journal covers the wide range of physical, biological and chemical sciences that underpin the design of biomaterials and the clinical disciplines in which they are used. These sciences include polymer synthesis and characterization, drug and gene vector design, the biology of the host response, immunology and toxicology and self assembly at the nanoscale. Clinical applications include the therapies of medical technology and regenerative medicine in all clinical disciplines, and diagnostic systems that reply on innovative contrast and sensing agents. The journal is relevant to areas such as cancer diagnosis and therapy, implantable devices, drug delivery systems, gene vectors, bionanotechnology and tissue engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


