通过碱介导的交叉共轭乙烯基苯并吡喃反应 (VBA) 快速合成 CF3-菲啶酮。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在此,我们报告了一种温和、高效、快速的方法,通过容易获得的 4-甲基-3-三氟乙酰喹啉酮和硝基烯烃的交叉共轭乙烯基[4 + 2]苯并反应制备 CF3-菲啶酮。与以往获得 CF3-菲啶酮的方法相比,本转化法更胜一筹,因为它完全是在简单碱的帮助下进行的,无需使用过渡金属催化剂或氧化剂。喹诺酮分子中的 CF3 基团具有很强的吸电子性,这促进了活性交叉共轭乙烯基烯醇的形成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
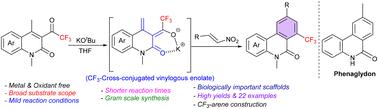
Expeditious synthesis of CF3-phenanthridones through a base-mediated cross-conjugated vinylogous benzannulation (VBA)†
Herein, we report a mild, efficient, and rapid approach for the preparation of CF3-phenanthridones through a cross-conjugated vinylogous [4 + 2] benzannulation of easily accessible 4-methyl-3-trifluoroacetylquinolones and nitro-olefins. The present transformation is superior to previous approaches for obtaining CF3-phenanthridones, in that it proceeds exclusively with the assistance of a simple base, eliminating the need for transition metal catalysts or oxidants. The strong electron-withdrawing nature of the CF3-group present in the quinolone moiety promotes the formation of a reactive cross-conjugated vinylogous enolate.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


